Lithothamnion muelleri is reported for the first time as one of the main components of rhodolith beds along the Eastern Pacific Ocean based on samples from Washington State (USA), Pacific Baja California (Mexico), southern Nicaragua, and Costa Rica. Individual rhodoliths ranged from fruticose to lumpy in morphology, and bi-sporangial, tetrasporangial, and gametangial plants were similar to those described from Australia and Brazil. Our study revealed a surprisingly wide latitudinal distribution of this species along the American continent. Its documentation in the Eastern Pacific will facilitate a more accurate interpretation of the ecology, biology, and biogeography of rhodolith beds worldwide.
Rhodoliths beds are widely distributed around the world (reviewed by Foster 2001). Recently, the species composition of these beds has been studied in the Gulf of California (Steller et al. 2009, Riosmena-Rodriguez et al. 2010), the northeastern Atlantic in Spain (Pena and Barbara 2010), the southwestern Atlantic in Brazil (Amado- Filho et al. 2007, 2010, Figueiredo et al. 2007, Villas-Boas et al. 2009, Henriques et al. 2012), and southern Australia (Harvey and Bird 2008). These accounts are part of an increasing interest in the taxonomy, distribution, and ecology of this poorly known habitat.
In the Eastern Pacific Ocean, rhodolith beds are known from the Galapagos Islands, Costa Rica, and Panama and from the Gulf of California to Alaska (Lemoine 1929, Dawson 1960, Konar et al. 2006, Fernandez 2008, Littler and Littler 2008, Riosmena-Rodriguez et al. 2010). However, characterization of the species forming these beds is still incomplete. Recently, a number of rhodolith collections from these sites became available for taxonomic study. Amazingly, most of them appeared to be a single widespread species (Fig. 1) with wide ecological tolerances. This species, occured in both intertidal and subtidal zones, at depths of up to 85 m, and was found growing on small stones, large rocks, and reef tops as well as on other algae (Wilks and Woelkerling 1995, p. 556, Womersley 1996, p. 183). In order to confirm that these collec-tions were truly a single species, we carried out a detailed morphological and anatomical study of the material from throughout its geographic range in the Eastern Pacific Ocean.
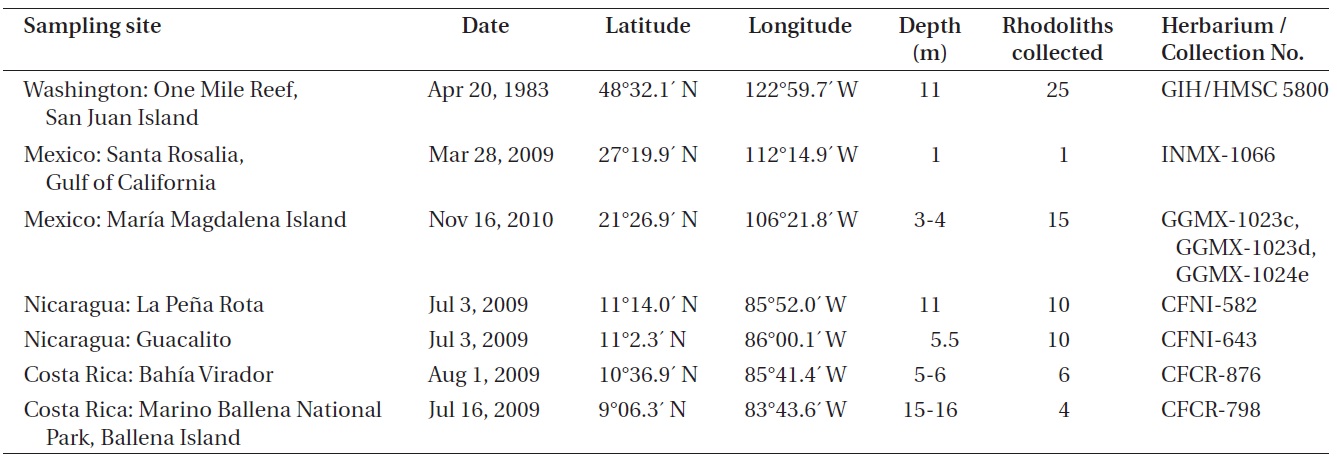
Collections used for the study came from seven sites in the Eastern Pacific Ocean (Table 1). Specimens were collected by snorkeling at depths of 1-4 m and by SCUBA diving at depths of 5-16 m. Anatomical measurements and reproductive features were determined for specimens from each collection, including a total of 71 rhodolith individuals and all possible morphological variants. Terminology for the growth forms of coralline algal thalli follows Woelkerling et al. (1993), and anatomical terminology follows Woelkerling (1988). Permanent slides for optical microscopy were prepared using the methodology described by Riosmena-Rodriguez et al. (1999). Specimens for scanning electron microscopy (SEM) were air-dried, fractured under the stereoscope, placed on aluminum stubs and then gold-coated for observation under the SEM (S-2600N; Hitachi, Tokyo, Japan), at 20 kV at the Ryan Institute, National University of Galway, Ireland.
The following collections were examined: GIH 5800, One Mile Reef, San Juan Island, San Juan County, Washington, USA, 11 m depth, April 20, 1983, collector (col.) S. Norton and G. Hansen, determiner (det.) R. Riosmena; CFNI-582 and CFNI-643, La Pena Rota and Guacalito, Nicaragua, 11 and 5.5 m depth respectively, July 3, 2009, col. C. Fernandez-Garcia, det. N. M. Robinson; GGMX-1023c, GGMX-1023d, GGMX-1024e, Maria Magdalena Island, Mexico, 3-4 m depth, November 16, 2010, col. G. Gallard and R. Riosmena, det. N. M. Robinson; INMX-1066, Santa Rosalia, Gulf of Califonia, Mexico, 1 m depth, March 28, 2009, col. R. Riosmena, det. N. M. Robinson; CFCR-876, Bahia Virador, Costa Rica, 5-6 m depth, August 1, 2009, col. C. Fernandez-Garcia, det. N. M. Robinson; CFCR-798, Marino Ballena National Park, Ballena Island, Costa Rica, 15-16 m depth, July 16, 2009, col. C. Fernandez-Garcia, det. N. M. Robinson.
The vegetative and reproductive anatomy of the specimens examined indicated that all thalli did fall within the morphological circumscription of
>
Lithothamnion muelleri Lenormand ex Rosanoff 1866 (
Lectotype. CN (herb. Lenormand), from Western Port Bay, Victoria, collected by W. H. Harvey 1851, communicated by F. Mueller, lectotype designed by Woelkerling 1983, p. 193, Fig. 1A.
Type locality. Western Port Bay, Victoria, collected by W. H. Harvey 1851, communicated by F. Mueller.
Illustrations of type material. Wilks and Woelkerling 1995, p. 572, Figs 1 & 2.
Heterotypic synonyms.
>
Vegetative morphology and anatomy
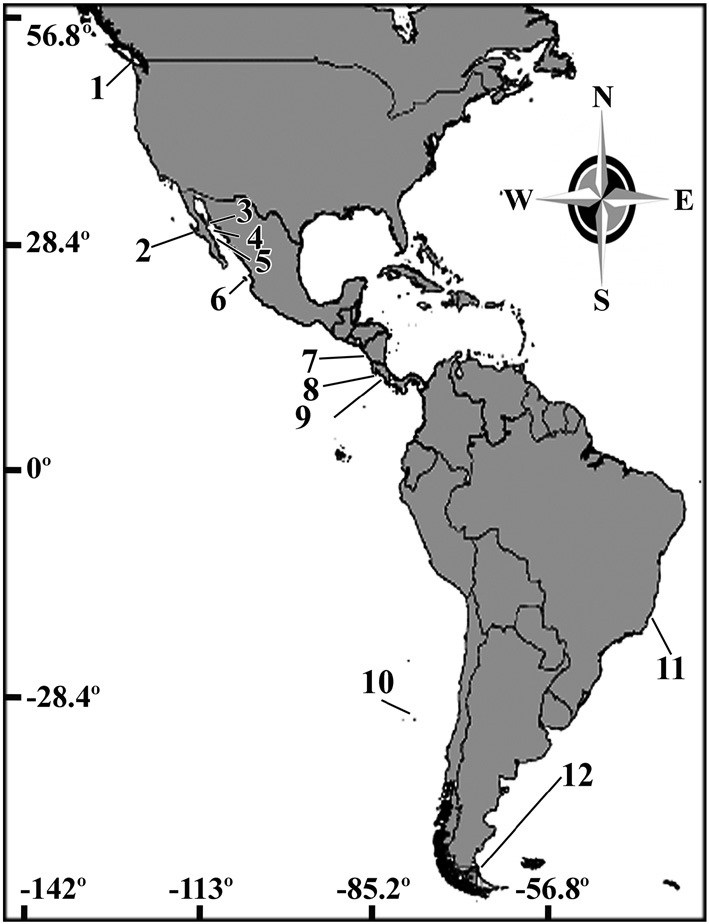
The vegetative structure of all specimens from the Eastern Pacific showed only slight variations. All thalli formed rhodoliths that were lumpy to fruticose in morphology
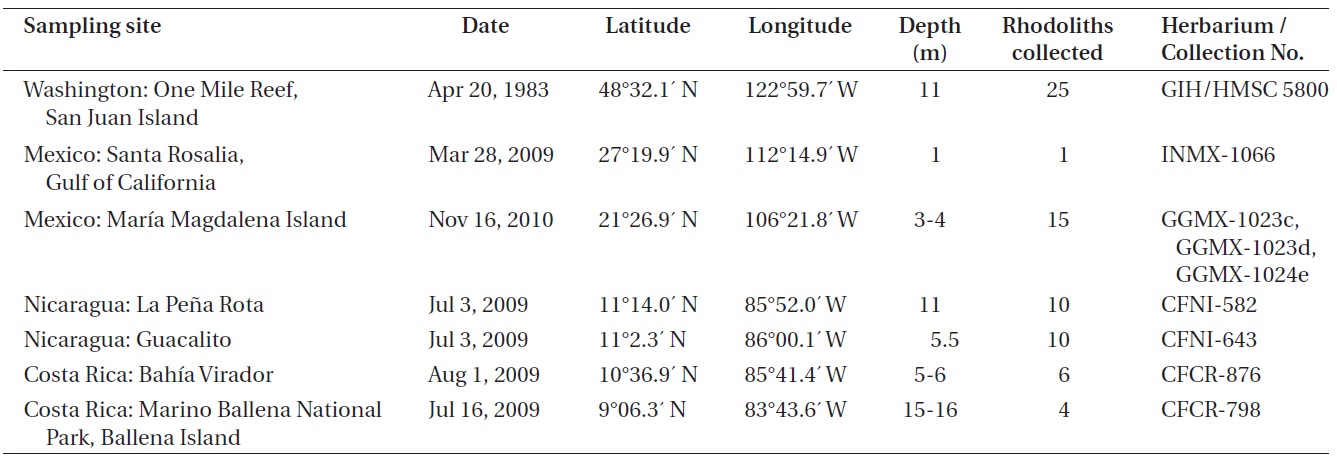
Geographical sites where rhodolith samples were collected in Washington, Mexico, Nicaragua, and Costa Rica
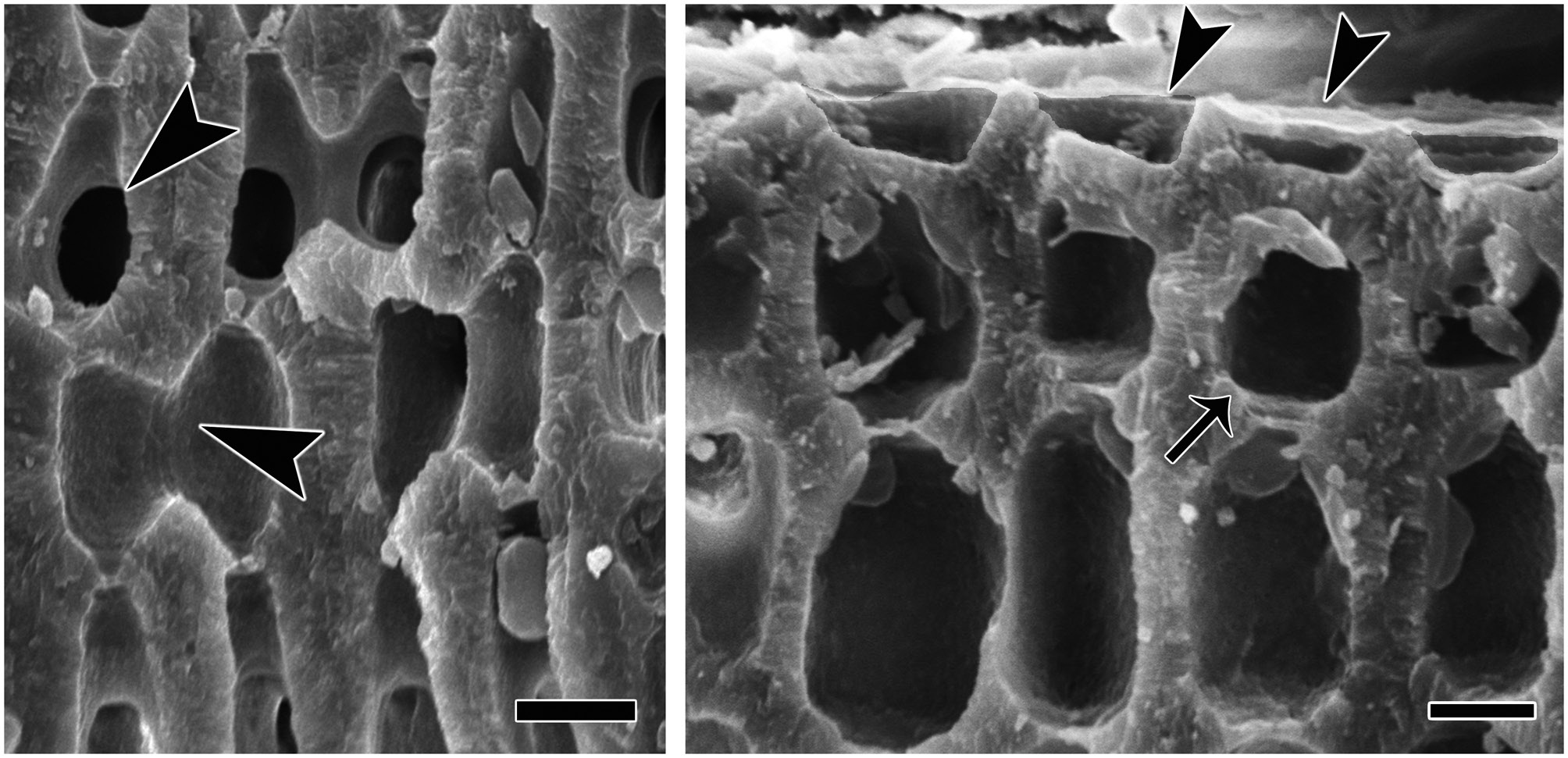
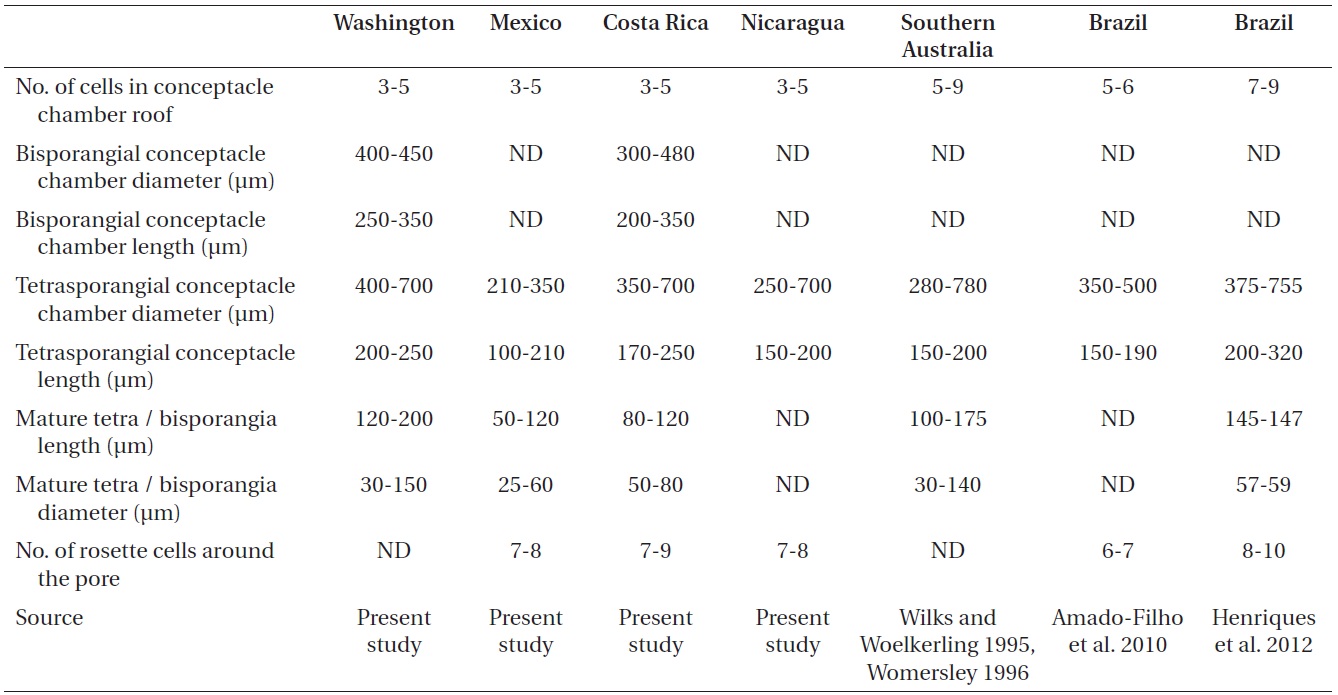
(Fig. 2). The protuberances were cylindrical and typically 5-7 mm in diameter and 2-8 mm long. All branches were pseudoparenchymatous with monomerous construction. Cells of the filaments were 10-30 μm long and 7-15 μm in diameter, and they were joined by cell fusions (arrowheads in Fig. 3A). In the epithallus filaments terminated in flared cells (arrowheads in Fig. 3B), 2-5 μm long and 7-10 μm in diameter. The subepithallial cells (arrow in Fig. 3B) were similar in length or shorter than their immediate inward derivatives. Trichocytes were absent. Clear growth bands were visible in many specimens. The monomerous internal structure of the rhodoliths consisted of a single system of branched filaments that formed a core and peripheral region. Portions of the core filaments and their derivatives curved outwards to the thallus surface forming a regular banding in the peripheral region.
The reproductive thalli, when present, also shared critical features (see Table 2). The bi and tetrasporangial thalli all had multiporate conceptacles with 20-60 pores per chamber, and in surface view each had a protruding roof that sometimes sank centrally leaving the margin elevated like a ring (Fig. 4A & B). In tetrasporangial conceptacles, the roof filaments bordering the pore canals (arrowhead in Fig. 4C) were composed of 3-5 cell layers of similar size and shape to other roof filament cells, and there was no depression around the pore. In surface view each pore was surrounded by 7-9 rosette cells which normally appeared collapsed and equal in size and shape to the other epithallial cells (Fig. 4D). Bispores, found only in Washington and Costa Rica, had dimensions similar to or larger than those known from Australian populations of this species. Carposporangial conceptacles were uniporate (Fig. 4E & F), and the roofs protruded above the thallus surface. Commonly, the conceptacle pores lacked senescent cells lining the pore (arrowhead in Fig. 4F). Spermatangial conceptacles were uniporate, and the roofs protruded above or were flush with the surrounding thallus surface. Branched spermatangial filaments arose from the floor and wall of the conceptacle chamber. In the type material, sterile cells were present within the conceptacle chamber. However, in our collections, these cells were either not evident or appeared degenerated due to tetrasporangial development or to poor preservation of the older collections.
There were some similarities and some surprising differences in the habitats of the collections. At One Mile Reef in Washington (located one nautical mile SE of the Friday Harbor Laboratories), the bed contained only one rhodolith-forming species,
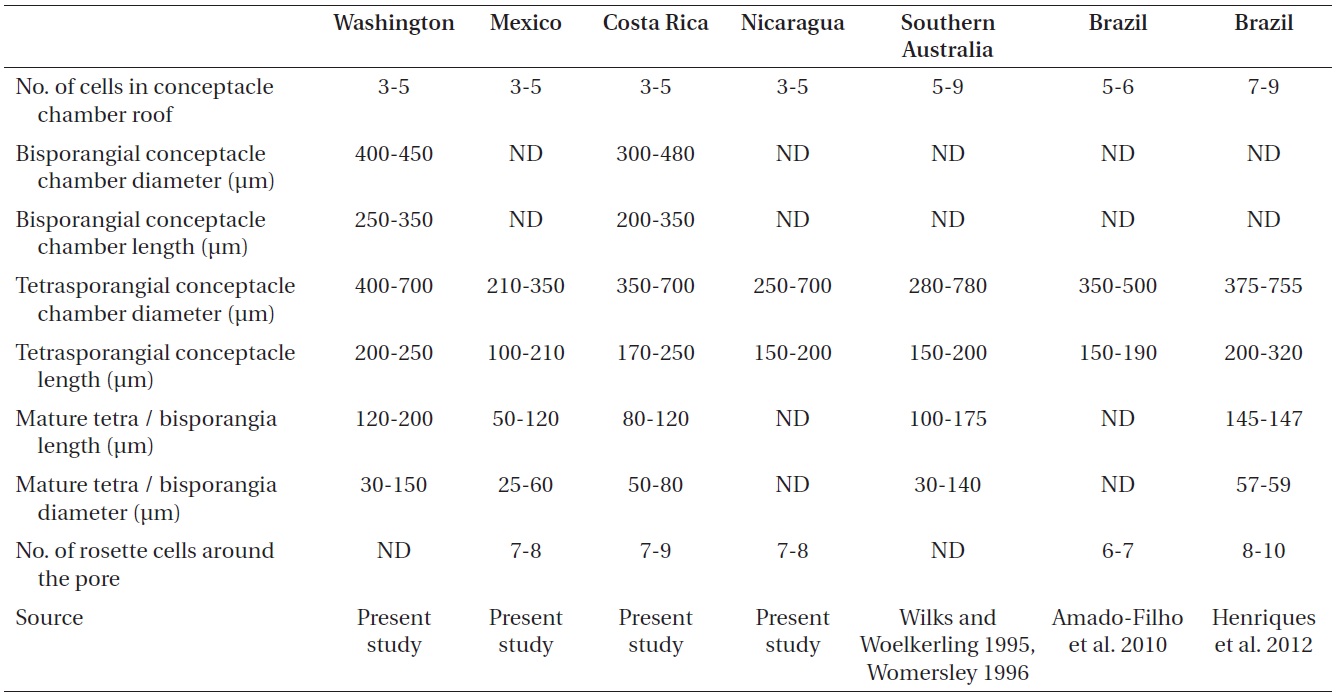
Morphometric reproductive characteristics of Liththamnion muelleri from populations in Washington, Mexico (Maria Magdalena Island), Costa Rica, Nicaragua, Southern Australia, and Brazil
The exceedingly wide range of this species makes us wonder if the taxonomy is correct and if there might be cryptic species involved such as those described in recent molecular studies (Broom et al. 2008, Walker et al. 2009, Bittner et al. 2011). Unfortunately, our material was not well enough preserved to use molecular methods to detect these species. So instead, we carefully examined the diagnostic morphological and life history features that were available in an attempt to shed light on variations that might offer clues to any hidden species. Most characteristics were quite uniform, but a few were variable.
>
Tetrasporangial roof anatomy
Wilks and Woelkerling (1995) considered characteristics of tetrasporangial roof anatomy as diagnostic for Australian species of
Instirosette cells around the pore area. We looked closely at the last feature. Athanasiadis et al. (2004) and Athanasiadis and Adey (2006) considered the characteristics of the rosette cells surrounding the pore area in multiporate roofs as a reliable and easily accessible feature for separating species in the genera
>
Growth-form differences in populations
In Australia, the crustose stage of
>
Life history differences in populations
The major life history difference we see in our collections of
Our collections and detailed morphological studies have documented the surprisingly wide latitudinal distribution of