Microalgae, as an evolutionary form of organism, are showing an extraordinary adaptation in the ocean. The oldest fossil evidence has shown that rise of environment oxygen that is contributed by eukaryotes and cyanobacteria appeared over 2.45-2.32 billion years ago (Rasmussen et al. 2008). In fact, blue-green algae (cyanobateria), diatoms (Bacillariophyta), and dinoflagellates (Dinophyceae) have evolved from their origins in a primitive age to contemporary times without much change in structure. It is this fascinating group of single-celled, microscopic organisms, which have been at the bottom of the food chain and have long served as a primary producer in aquatic sources either fresh or marine, contributing to almost 70% of global oxygen demand, even though abiotic factors such as, light intensity, temperature, nutrients, and salinity levels influence their biological functions. Furthermore, extreme fluctuations in climate changes according to the seasons have given a major outbreak through the variations and required for survival in the competitive environmental conditions (Plaza et al. 2008).
As a consequence, biochemically and ecologically significant differences have been gained a vast microalgae diversity and associated a broad spectrum of secondary metabolites. Thus, researchers are continuously mining the bioactive components from marine microalgae to determine pharmacological and medicinal values in many parts of the world (Borowitzca 1995). Hence, marine microalgae have emphasized that research on their natural products are useful for the cure and for the alleviation of human diseases (Imhoff et al. 2011). Screening of marine microalgae has received much attention as a rich source for the exploration of pharmaceutical and other biological active components in last decades (Borowitzca 1995). In this regard, microalgae bear an unusual quality of nutrients that include a rich source of protein, poly unsaturated fatty acids, carbohydrates, minerals, vitamins, pigments, and secondary metabolites (Becker 2007). Thus, many of the prominent microalgae natural sources have targeted for the research and revealed the health effects, for human well-being (Liang et al. 2004) as well as for domestic animal applications (Becker 2007). However, there are some potential benefits in addition to the nutritional value of microalgae, including antioxidant (Sheih et al. 2009), antitumor (Sheih et al. 2010), immunostimulant (Morris et al. 2007), antibacterial (Desbois et al. 2009), hypocholesterolemic effect (Dvir et al. 2009), and angiotensin- converting enzyme inhibitor-I inhibition activity (Samarakoon et al. 2013).
The cultivation of marine microalgae has been practiced for some time. In particular, there can be described a couple of algae systems that culture as open, closed (photobioreactors) or artificial culture. Each one of the culturing systems has either a desirable or undesirable effect, since the photosynthetic organisms and their mass production may directly correlate with the source of energy. Nevertheless, only a select few microalgae strains are used in controlled conditions for cultivation in particular environments (Hong et al. 2012). The controlled optimum condition with the operational inputs such as salt, dissolved CO2, water, nutrients, pH, and O2 providing a great opportunity for the steady environment without being contaminated in photobioreactors (Plaza et al. 2009). Hence, researchers have been interested in cultured marine microalgae in order to reveal the biochemical constituents of their crude extracts and to determine which components have pharmacological effects (Kim and Wijesekara 2010).
In this study, four different microalgae strains belonging to the three main classes of marine microalgae including cyanobateria (
The murine macrophage cell line (RAW 264.7), a human promyelocytic leukemia cell line (HL-60), a mouse melanoma cell line (B16F10), and a human lung carcinoma cell line (A549) were purchased from the Korean Cell Line Bank (KCLB, Seoul, Korea). Roswell Park Memorial Institute (RPMI-1640) medium, Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), and penicillin-streptomycin were purchased from Gibco/BRL (Burlington, ON, Canada). 3-(4,5-Dimethylthiazol- 2-yl)-2,5-diphenyltetrazolium bromide (MTT) and dimethyl sulfoxide (DMSO) were purchased from Sigma- Aldrich (St. Louis, MO, USA). All other chemicals and reagents used in these investigations were of analytical grade.
>
Culture of marine microalgae
Marine microalgae
Collected benthic dinoflagellate strains from different coastal sites of Jeju Island and established as single cell cultures. The culture of
>
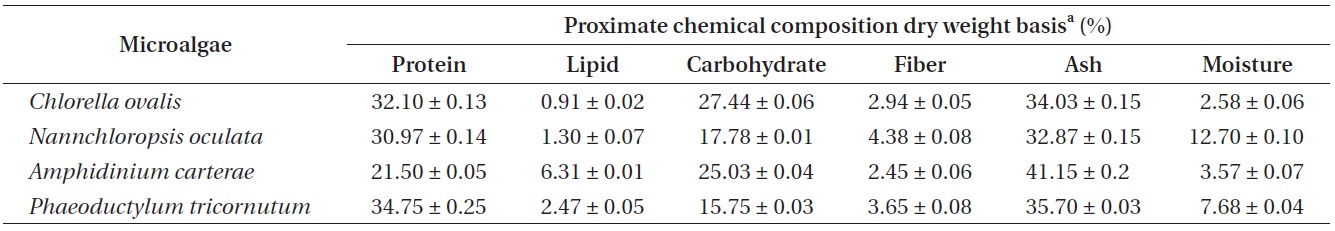
Determination of proximate chemical compositions of the cultured marine microalgae
Proximate chemical composition of cultured marine microalgae was determined according to the Association of Official Analytical Chemists (AOAC) method (AOAC 1990). Total nitrogen content was analyzed by the Kjeldahl method (Kejltec 8400; FOSS, Eden Prarie, MN, USA). Crude protein was determined by calculating a conversion factor of 6.25. Crude lipid was performed by soxhelt method extraction with diethyl ether solvent (Soxtec 2050; FOSS) and crude ash was determined by incineration of samples at 550℃ in the muffle furnace (B180; Nabertherm GmbH, Lilienthal, Germany). Moisture was determined by oven-drying method at 105℃ in the moisture analyzer (mb45; OHAUS, Nanikon, Switzerland). Crude fiber content was measured by fibertec system (Fibertec 2010 Analyzer; FOSS).
>
Solvent extractions and preparation of samples from the cultured marine microalgae
After freeze-drying, the cultured marine microalgae were grounded into fine powder and each of the materials was homogenized separately. Then, the homogenized marine microalgae samples were sonicated at 25℃ for 90 min, 3 times each using (80%) methanol. Crude methanol extracts were concentrated by evaporating the solvent under reduced pressure using rotary evaporator and further subjected to solvent-solvent partition chromatography. Four different fractions such as
HL-60 (a human promyelocytic leukemia cell line) and A549 (a human lung carcinoma cell line) cells were grown in RPMI-1640 medium, B16F10 (a mouse melanoma cell line), and RAW 264.7 (a murine macrophage cell line) were cultured in DMEM. Both culture media were supplemented with 100 U mL-1 of penicillin, 100 μg mL-1 of streptomycin and 10% FBS. The cells were incubated and maintained in an atmosphere of 5% CO2 at 37℃. The cells were subcultured every 2 days and exponential phase cells were used throughout the experiments.
>
Determination of nitric oxide (NO) production
RAW 264.7 cells (1 × 105 cells mL-1) were placed in a 24-well plate and after 24 h the cells were pre-incubated with various concentrations of the sample at 37℃ for 1 h. Then further incubation was done for another 24 h with lipo-polysaccharide (LPS; 1 μg mL-1) at the same temperature. After the incubation, a quantity of nitrite that had accumulated in the culture medium was measured as an indicator of NO production (Lee et al. 2007). Briefly, 100 μL of cell culture medium was mixed with 100 μL of Griess reagent (1% sulfanilamide and 0.1% naphthylethylenediamine dihydrochloride in 2.5% phosphoric acid), the mixture was incubated at room temperature for 10 min, and the optical density at 540 nm was measured using
[Table 1.] Proximate chemical compositions of marine microalgae sample crude dry weight basis
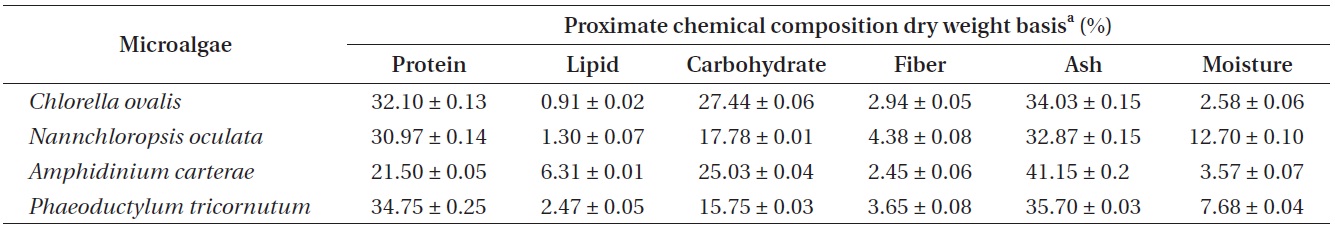
Proximate chemical compositions of marine microalgae sample crude dry weight basis
an enzyme-linked immunosorbent assay (ELISA) microplate reader (Sunrise; Tecan Co. Ltd., Salzburg, Australia). The fresh culture medium was used as a blank in every experiment.
>
Cytotoxicity assessment using MTT assay
The cytotoxicity of the crude solvent extracts against the RAW 264.7 cells was determined using the colorimetric MTT assay. Cells were seeded in a 24-well plate at a concentration of 1 × 105 cells mL-1. After 24 h, the seeded cells were treated with extracts. Then, all of the cells were incubated for an additional 24 h at 37℃. MTT stock solution (50 μL; 2 mg mL-1 in phosphate-buffered saline [PBS]) and was added to each well to a total reaction volume of 250 μL. After incubating for 3 h, the supernatants were aspirated. The formazan crystals in each well were dissolved in 200 μL of DMSO. The resulting absorbance was measured with an ELISA plate reader set at 540 nm.
>
Cell growth inhibitory assay for anticancer activity
The cell growth inhibitory activity of cultured marine microalgae (
All the data are expressed as mean ± standard deviation of three determinations. Statistical comparison was performed via a one-way analysis of variance followed by Duncan’s multiple range test (DMRT). p-values of less than 0.05 (p < 0.05) were considered as significant.
>
Proximate chemical composition and solvent extraction of the cultured marine microalgae
According to the AOAC (1990) method, the proximate chemical composition (%) of the cultured marine microalgae samples were determined by crude dry weight basis. In this study, two cyanobacteria species (
and
to isolate the possible bioactive lipids than other cultured species. In addition, a recent publication has been reported that the high content of proteins as the range of 40-60% of their dry weight in marine microalgae, which included
Solvent-solvent partition chromatography was subjected to fractionation of the crude samples of (80%) methanol extracts further from the cultured marine microalgae as shown in Fig. 1. The chemistry of this approach was based on the variation in organic solvent polarity to separate different functional metabolites in the crude solvent extracts. Therefore, more significant exploration was achieved with the organic solvent fractionations in order to gain a different polarity of crude extracts from the cultured marine microalgae samples.
>
Anti-inflammatory effect of the extracts and fractions from cultured marine microalgae
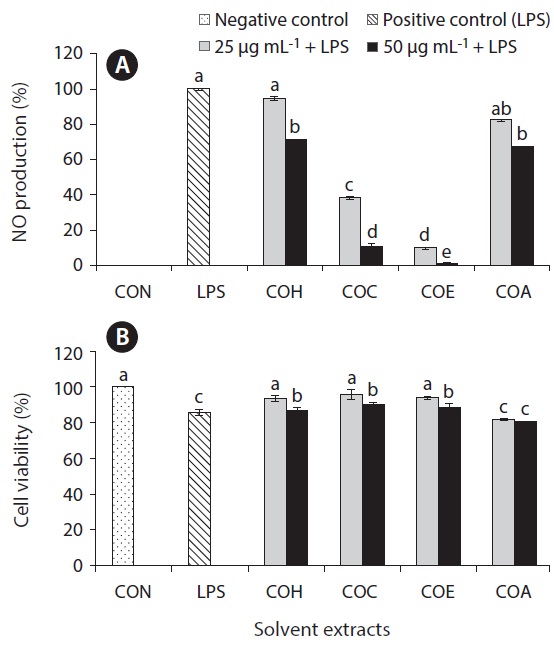
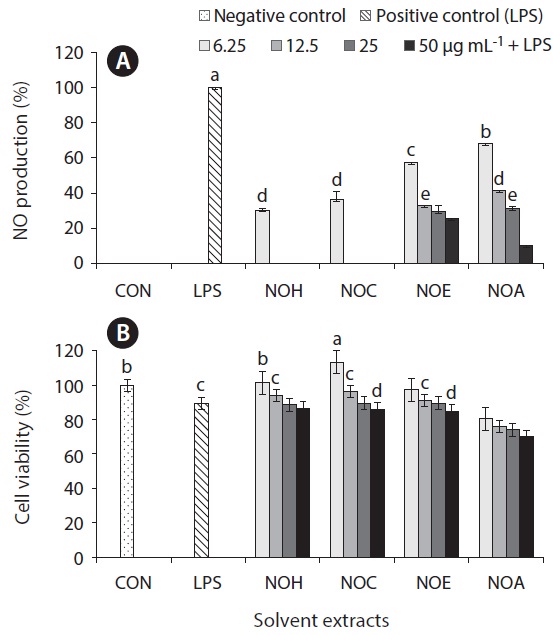
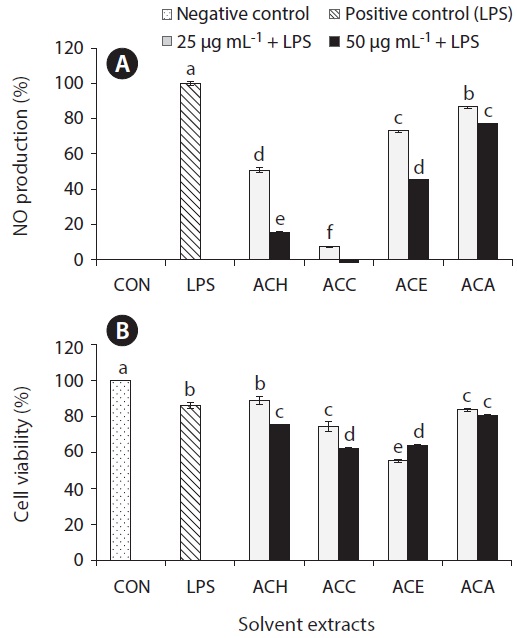
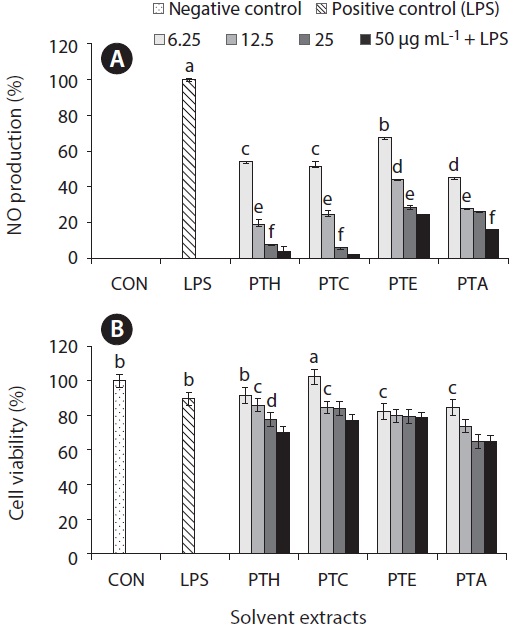
Inflammation is a physiological process that is initiated due to the pathogenic invasion or injury to cells and tissues (Wadleigh et al. 2000). This can be influenced by the activation of various immune cells such as macrophages, neutrophils and lymphocytes. Inflammatory mediators, such as NO, play an important role as the signaling molecules that are induced in macrophages. LPS acts as endotoxins for mammals and stimulation of the RAW cells in terms of enhancing the NO concentration in the medium. Hence, the inhibition of NO production (%) level was indicated that the positive evidence of anti-inflammatory activity on LPS-induced RAW macrophages. Figs 2A, 3A, 4A, and 5A are showing the inhibitory effect of NO production
level of marine microalgae including
extracted from the cultured marine microalgae (Figs 2, 4 & 5), respectively.
In addition, the cytotoxic effects on RAW 264.7 cells with the treated samples were performed by MTT assay. The cell viability (%) of the extracted samples from the cultured marine microalgae was shown in the Figs 2B, 3B, 4B & 5B. According to the results, NOH and NOC fractions were reported to have the high cell viability against RAW 264.7 cells compared to the other fractions followed by LPS induced toxicity. More importantly, the concentration of NOC at 6.25 μg mL-1 was shown the highest cell viability as nearly 120% compared to the negative control. In addition, same the concentration of PTC and NOH are also reported more than 100 (%) of cell viability with comparatively significant differences. The fractions of
>
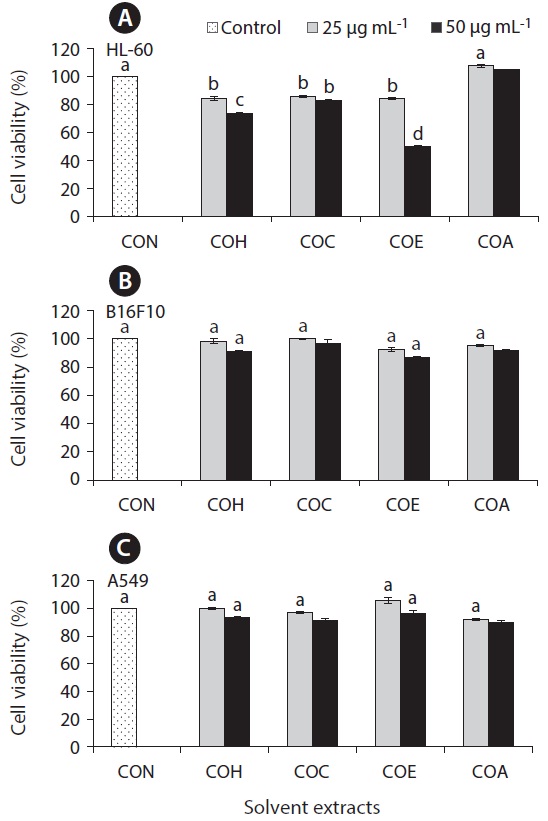
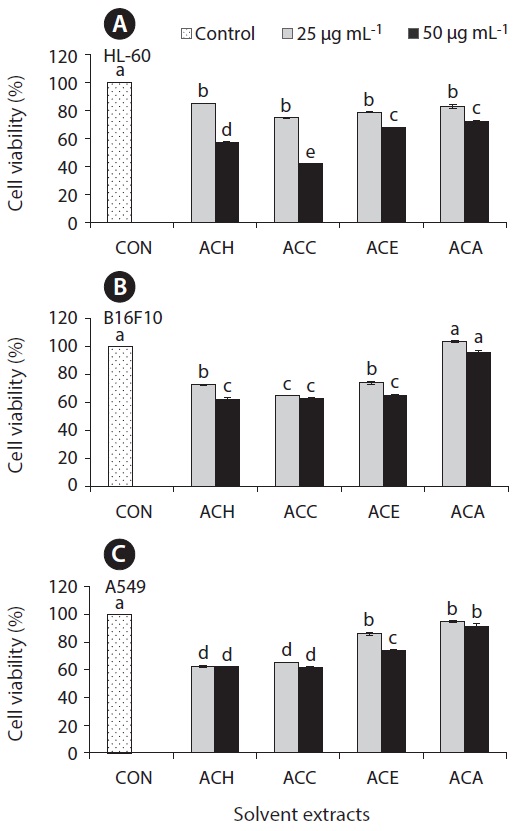
Anticancer effect against HL-60, B16F10, and A549 cell lines
The cell growth inhibitory effect was examined against different cancer cell lines followed by pre-treated cultured marine microalgae solvent extracts and fractions for screening anticancer activity of the samples. Figs 6 and 7 represent the cell viability against treated samples from cultured marine microalgae including
In the present study, four different marine microalgae species including