Heterotrophic microalgal species are limited in number and their culture methods are not as fully known as autotrophic microalgal species (Olaizola 2003). Although heterotrophic culture of microalgae has advantage for pure high-density culture due to no photoinhibition effect and easy harvest, the cost of initial investment is very high. Therefore, they need to be high value species that can grow rapidly in a fermenter without light (Barclay et al. 1994, Wen and Chen 2003).
Chlorella, a typical genus that can be heterotrophically cultured, has a high growth rate and is looked forward to as a future food as it contains a considerable amount of protein, dietary fiber, vitamin, and mineral, etc. (Kim 2004). A chlorella growth factor (CGF), in particular, is known to be effective in growth and recovery after illness (Chen 1996, Lee 1997). Chlorella has been also reported to have anticarcinogenic, antioxidative, and skin-lightening effect (Hasegawa et al. 1994, Justo et al. 2001, Kim et al. 2008). In addition, it is in the spotlight as an ingredient of biodiesel since it contains a variety of lipid composition (Miao and Wu 2004, Miao et al. 2004, Xiong et al. 2008).
Although many results have been reported in terms of heterotrophic culture of Chlorella (Shi et al. 1999, 2002, Xiong et al. 2008), new Chlorella species with outstanding growing ability under heterotrophic culturing-condition should be developed more for industrial purpose. In this study, the optimum culture conditions such as carbon source, temperature, and nutrient of three heterotrophic Chlorella species that have been collected from Korean waters were examined on growth rate. And biomass and fatty acid composition of heterotrophically cultured Chlorella and autotrophically cultured one were compared each other.
Two strains (C4-3 and C4-4) of C. vulgaris and one Chlorella sp. (C4-8) were received from Korea Marine Microalgae Culture Center (KMMCC) in Pukyong National University. They were cultured in axenic condition. As for culture, a 100 mL sized-standing culture method was used with a Jaworski’s medium (JM) (Thompson et al. 1988) at 25℃ with continuous light of 60 mol photons m-2 s-1 in a 250 mL flask. The cells in exponential growth phase were used as inocula.
Three organic matters, glucose, glycerol, and acetate were tested as carbon sources. Ten mililiters of Chlorella, which was autotrophically cultured in axenic state was inoculated to 100 mL of JM medium in a 250 mL flask. The initial cell density was 106 cells mL-1 and each carbon source with 1 and 2% concentration was added. Then, they were cultured under a continuously dark condition at 28℃ for 6 days with three repetitions. The number of Chlorella cells was counted three times a day at the same time using a haemacytometer and the most ideal carbon source for heterotrophic culture was determined by comparing specific growth rate [SGR = 3.322 log(N2/N1)/(t2 - t1); N1, a number of cells at t1 culture starting date; N2, a number of cells at t2 the highest cell density date] (Guillard 1973).
The SGR of Chlorella with different temperatures (24, 26, 28, 30, and 32℃) and glucose concentrations (1, 2, 3, 4, and 5%) were examined. The experiment at 32℃ was conducted only on the species that grew at 30℃. The other experimental methods were the same as before. To find the optimal concentration of nitrogen and phosphorus for heterotrophic Chlorella, the concentrations of Ca(NO3)2 (0.02 g L-1) and NaNO3 (0.08 g L-1) as a nitrogen source and KH2PO4 (0.0124 g L-1) and Na2HPO4 (0.036 g L-1) as a phosphorus source in JM medium, were adopted as a control plot, respectively. There were seven different experimental plots that were prepared by setting nitrogen and phosphorus concentration as double and triple that of the control plot, and adding the control, doubled, and tripled amount of phosphorus when nitrogen is doubled and tripled, respectively. Chlorella was heterotrophically cultured with different concentrations of nitrogen and phosphorus in a medium added 1% glucose. The other experimental methods were the same as before.
The SGR and fatty acid composition of Chlorella that were cultured in heterotrophic or autotrophic condition were examined. For heterotrophic culture, 1% glucose was added and dark culture method was used at 26℃. For autotrophic culture, a light with 60 μmol photons m-2 s-1 was continuously supplied, replacing glucose. The rest of the procedures for experimental methods remained the same. The size of Chlorella from 100 cells selected randomly was measured with Moticam Pro 205A (Motic Instruments Inc., Richemond, BC, Canada). For dry weight measurement, 1.2 μm glass microfiber filter (GF/C; Watman, Kent, UK) was dried at 60℃ for 2 h and its weight was measured. The cells from 25 mL of culture medium were harvested through filtration and dried via the same method. The biomass in dry weight of cells was measured repeatedly for three times.
For analysis of fatty acid, Chlorella was cultured and harvested in the same manner as above and kept at -80℃ until the time of analysis. Fatty acid methyl esters (FAME) were prepared by trans-esterification with 10% BF3-MeOH at 85℃ for 1 h (Morrison and Smith 1964). A gas chromatography (HP 6890N; Agilent, Santa Clara, CA, USA) equipped with an Auto Sampler (Agilent) was used for fatty acid analysis. A w-wax column (30 m long, 0.25 mm id, 0.25 μm film thickness; Supelco, Bellefonte, PA, USA) was used for separations. Nitrogen was used as the carried gas and the flow rate was set at 30 mL min-1. The column temperature profile was same as the follows: standing at 200℃ for 3 min, increase to 1℃ min-1 from 200 to 230℃, and then hold at 230℃ for 25 min. Temperature of injector was 250℃ and flame ionization with the detector (FID) was held at 250℃. Fatty acid peaks were integrated using the GC (HP 6890N). A gas chromatography software and identification was made with reference to known standards (PUFA 37 component FAME Mix; Supelco).
The results of this study were analyzed by one-way ANOVA and Duncan’s multiple range test (Duncan 1955) was applied for the significance level (p < 0.05). The SPSS version 17 (SPSS Inc., Chicago, IL, USA) program was used for all statistical analyses.
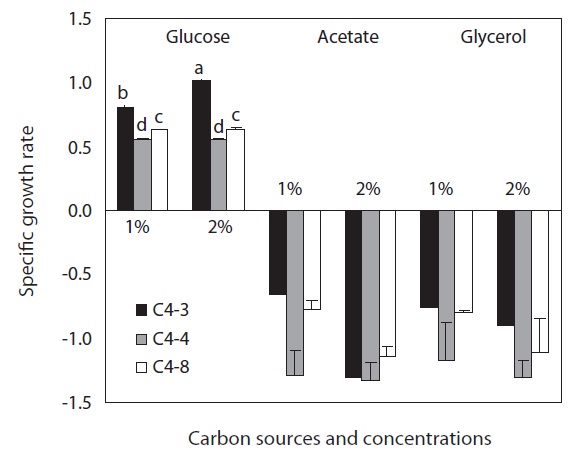
As a result of culturing Chlorella through heterotrophic method using three different carbon sources, it was determined that glucose was a proper carbon source since no growth was confirmed in the experimental plots using glycerol and acetate. In glucose, all three Chlorella species showed growth rate above 0.5 in 1% and 2% concentration. C. vulgaris (C4-3) showed a higher growth rate compared to the other species and its growth rate was significantly higher in the 2% than 1% glucose. However, no significant difference was observed in glucose concentration of the other species (Fig. 1).
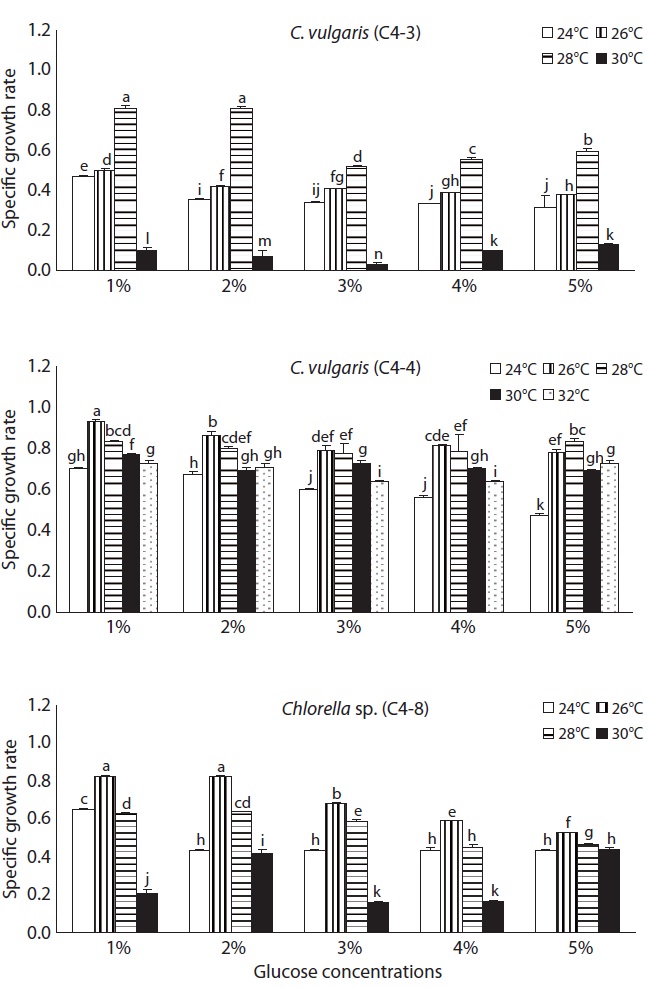
The growth rate influenced by temperature and glucose concentration is shown in Fig. 2. As the temperature elevated from 24 to 28℃, the growth rate of C. vulgaris (C4-3) was significantly enhanced with all different glucose concentrations, but it rapidly decreased to 0.1 at 30℃. A higher growth rate was observed in the experimental plot in which 1% or 2% glucose was added than when 3-5% glucose was added. The highest growth rate (0.81) was observed at 28℃ with 1% or 2% glucose concentration. C. vulgaris (C4-4) showed the highest growth rate among three species, and particularly, the growth rate at 32℃ was over 0.6. The growth rate of this species at 32℃ was about the same as that of 24℃ or 26℃, which suggests that this species is more thermophilic and eurythermal than the other two species. The growth rate in 1-2% glucose tended to be higher than that in 3-5% glucose. The growth rate of Chlorella sp. (C4-8) was always
higher at 26℃, regardless of the glucose concentration. The highest growth rate (0.82) was in 1% and 2% glucose at 26℃ (p < 0.05) and the rate decreased significantly at 30℃ as C. vulgaris (C4-3).
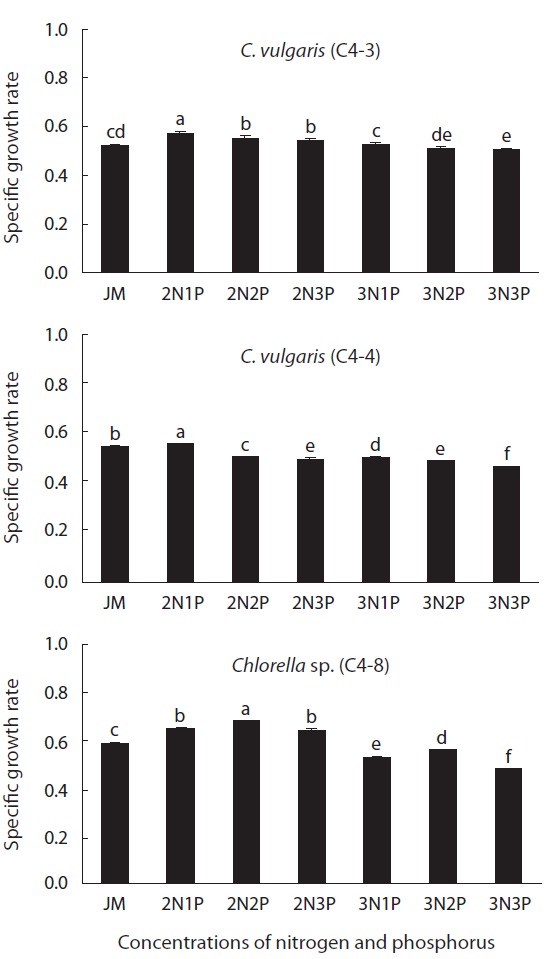
The effect of nitrogen and phosphorus concentration on the growth rate of Chlorella is illustrated in Fig. 3. The growth rate of two strains (C4-3 and C4-4) of C. vulgaris was 0.57 and 0.56, respectively, in doubled concentration of nitrogen sources [Ca(NO3)2 and NaNO3] of JM medium. The growth rate was significantly increased by double
that of the original medium (p < 0.05). However, in case of Chlorella sp. (C4-8), the highest growth rate (0.68) was in a medium containing a doubled concentration of nitrogen and phosphorus sources (p < 0.05). When the concentration of nitrogen was increased twofold, Chlorella sp. (C4-8), showed no significant difference between the experimental plot containing phosphorus of the original concentration and the plot containing phosphorus whose concentration was tripled. Therefore, it was determined that this species was more adaptable to phosphorus concentration compared to the other two species. The growth rate of all three species was the lowest when the concentration of both nitrogen and phosphorus was triple that of JM medium (p < 0.05).
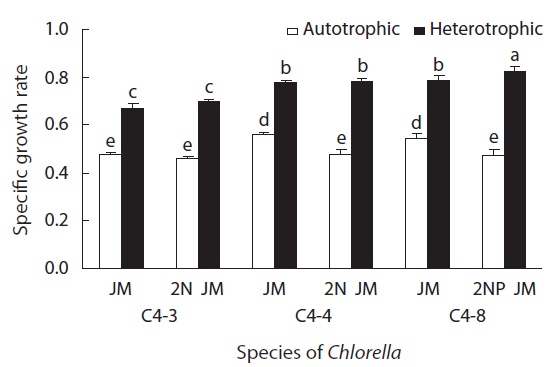
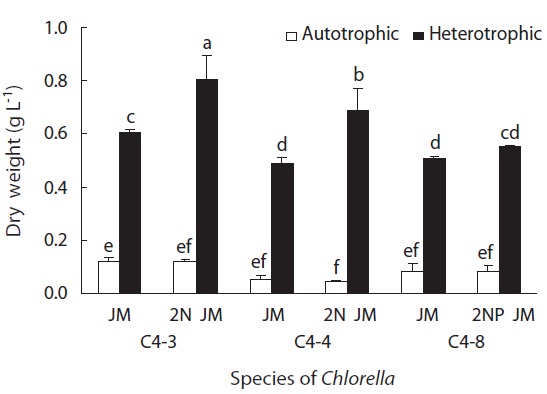
In all experimental plots, the growth rate of heterotrophically cultured ones was significantly higher than that of autotrophically cultured ones (p < 0.05). In heterotrophic culture, the growth rate of all three species was higher in doubled concentrations of nitrogen or phosphorus than in the control JM. But for autotrophic culture, the growth rate was rather higher in the control JM. This suggests that the required concentrations of nitrogen and phosphorus are different in heterotrophic and autotrophic culture (Fig. 4).
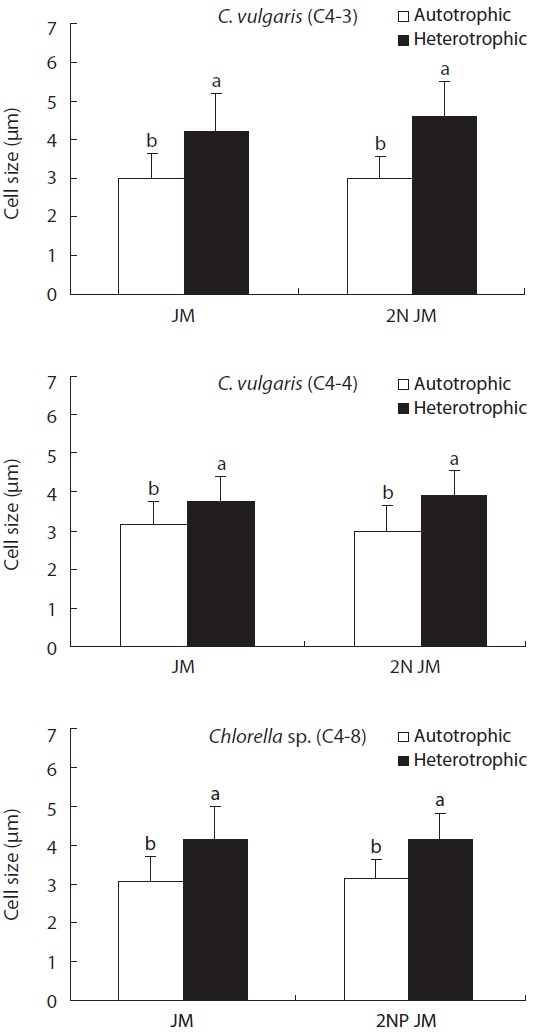
On the other hand, the size of cell was much bigger in heterotrophic culture than in autotrophic culture in all experimental plots (p < 0.05). In addition, the concentrations
of nitrogen and phosphorus did not have a much impact on the size of cell (Fig. 5). The biomass in dry weight of three species is shown in Fig. 6. In all experimental plots, the biomass in heterotrophic culture was significantly higher than that of autotrophic one (p < 0.05). The dry weight of two strains (C4-3 and C4-4) of C. vulgaris culture with doubled concentration of nitrogen was significantly higher than that of the control JM. But no significant difference was observed in Chlorella sp. (C4-8). Among three species, the biomass in dry weight of C. vulgaris (C4-3) was the highest with 0.8 g L-1.
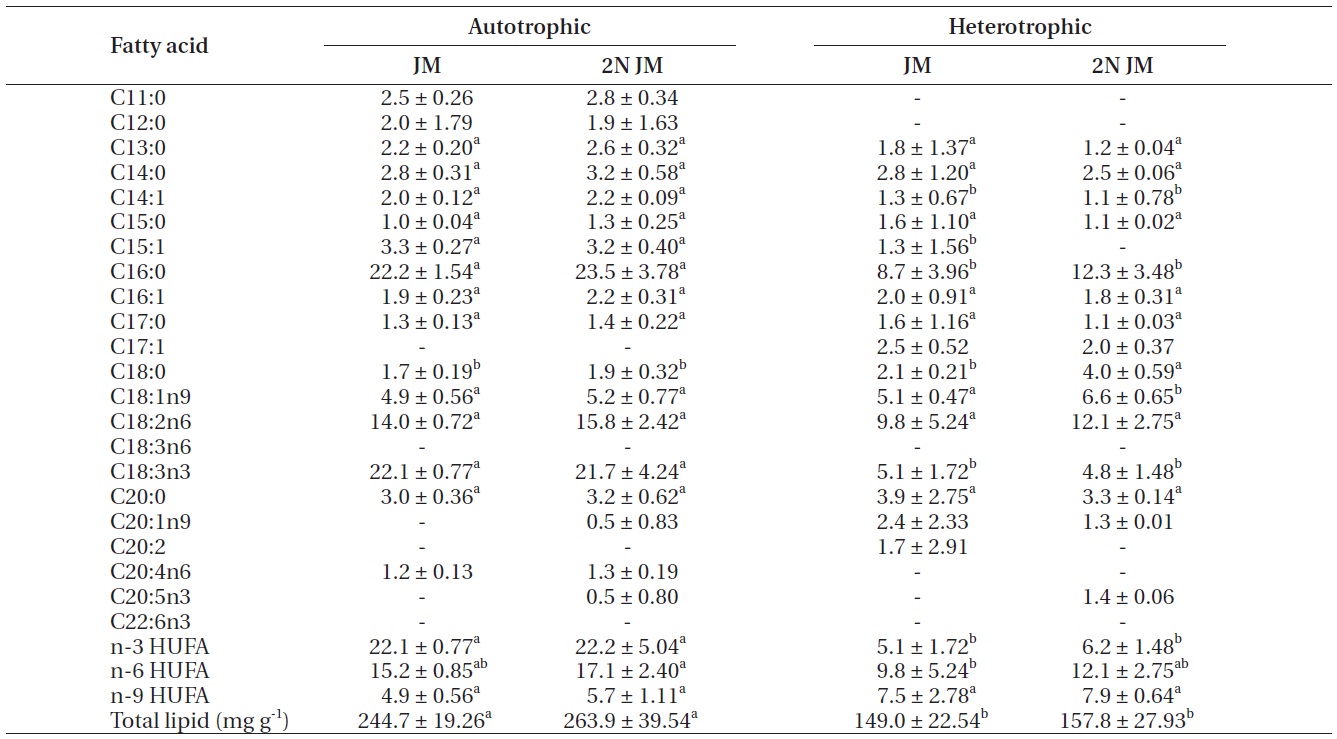
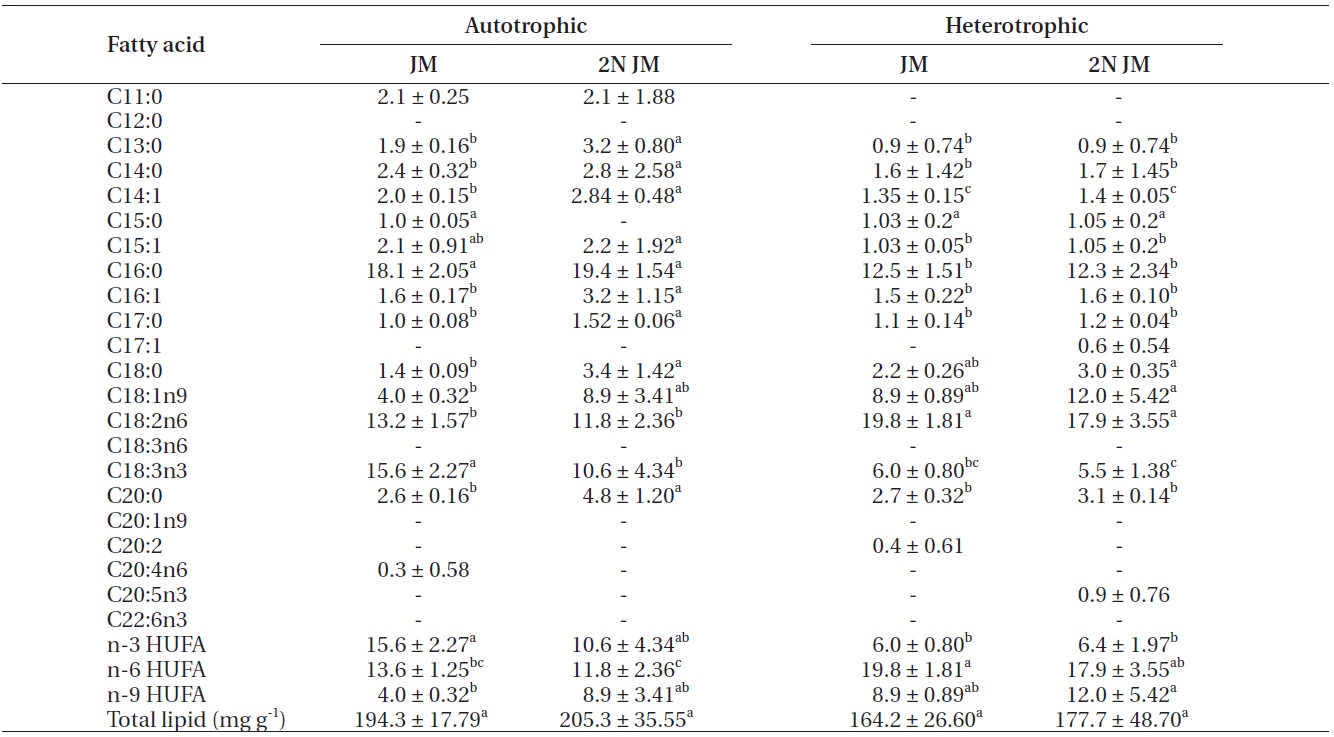
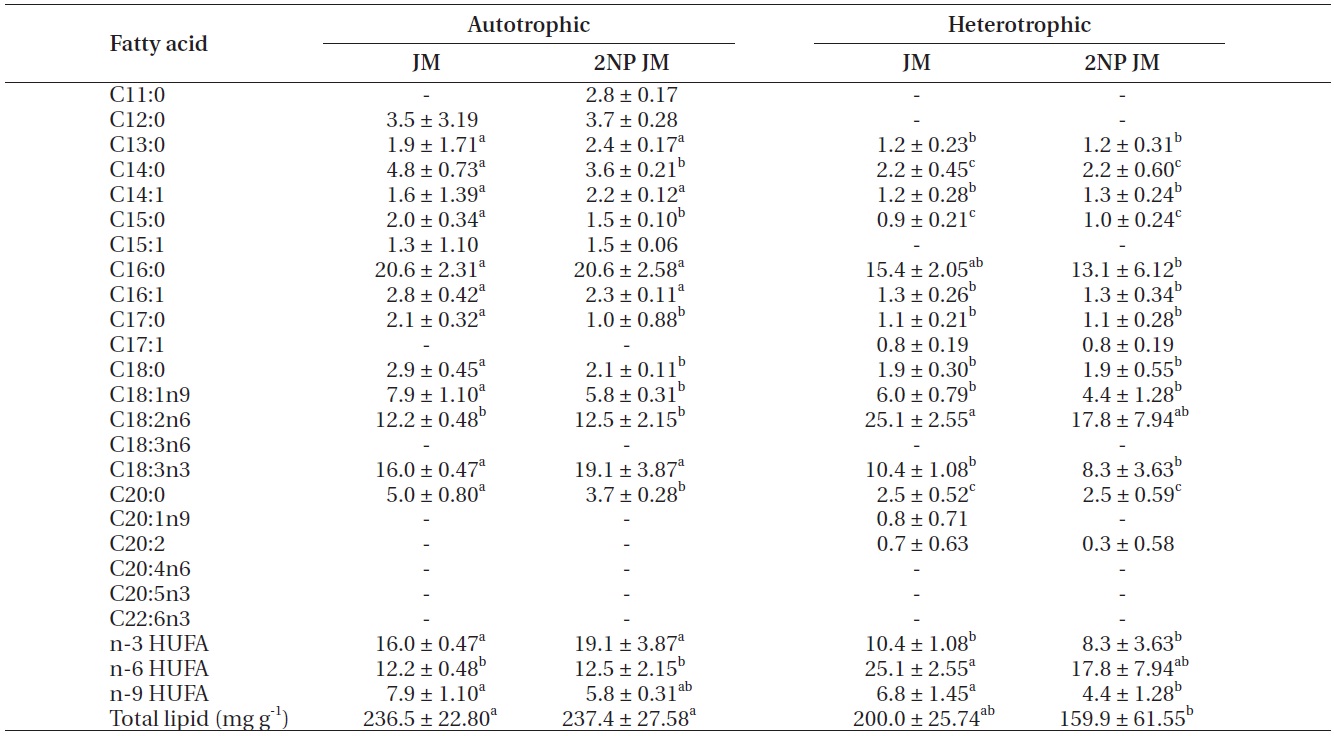
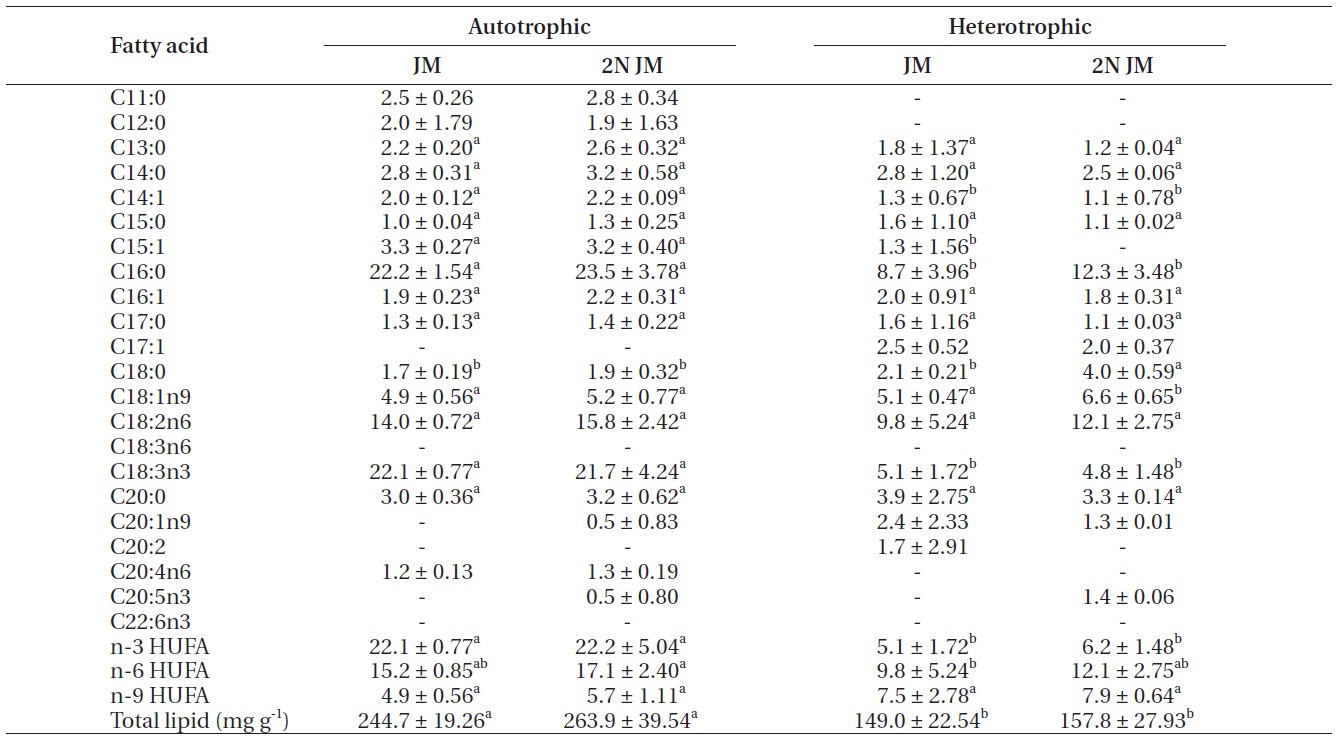
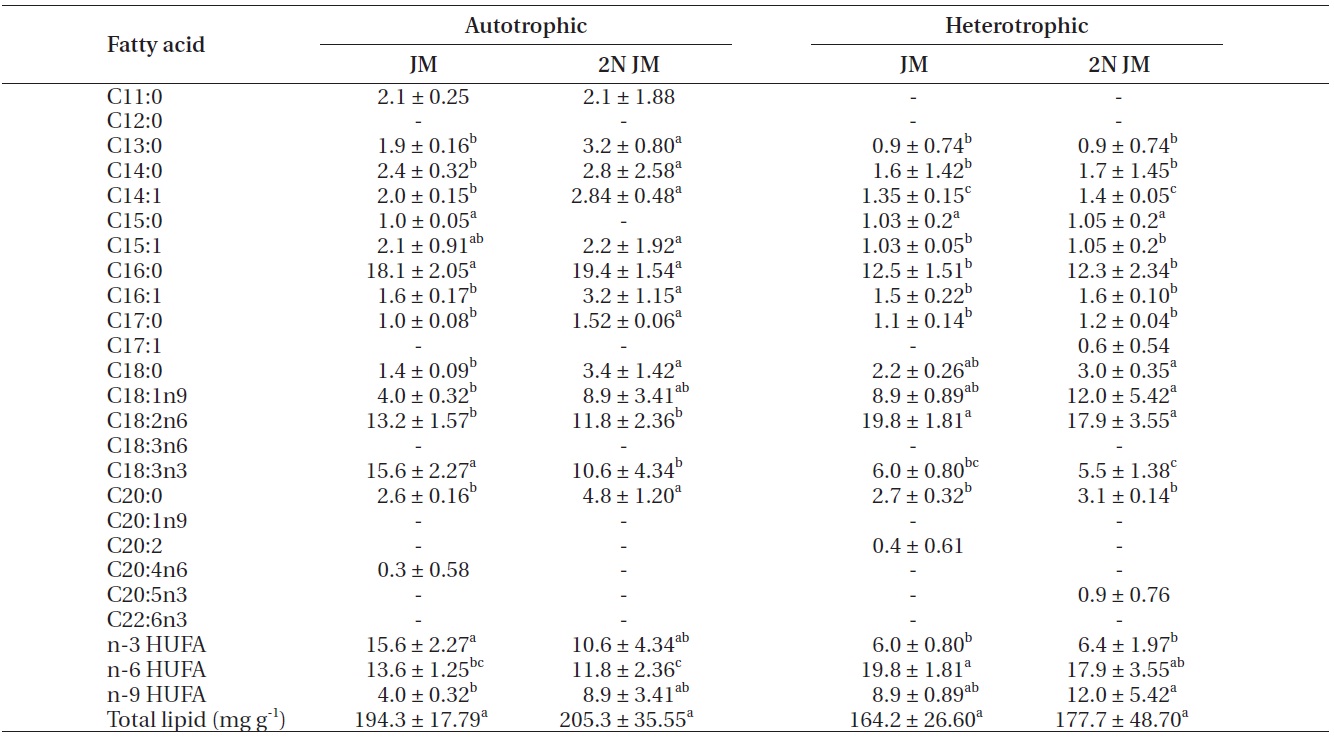
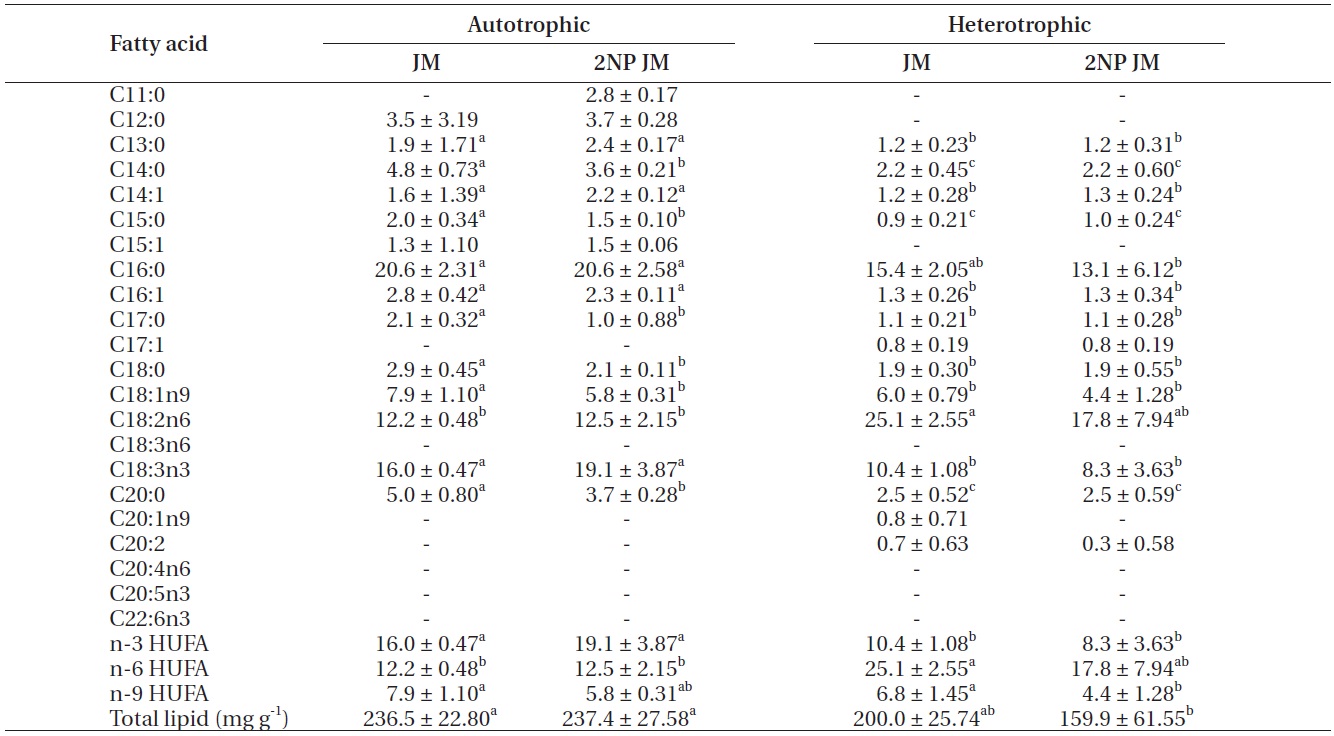
The fatty acid composition of Chlorella species cultured autotrophically and heterotrophically is shown in Tables 1-3. The fatty acid content of C16:0, C18:2n6, and
C18:3n3 was the highest in all three species. In case of C16:0 and C18:3n3, the composition of autotrophically cultured one was much higher than that of heterotrophically cultured one (p < 0.05). As for C18:2n6, there was not any difference in C. vulgaris (C4-3), but the results of C. vulgaris (C4-4) and Chlorella sp. (C4-8) were considerably higher with heterotrophic culture. The concentration of nitrogen did not seem to have much impact on fatty acid composition.
In this study, docosahexaenoic acid (C22:6n3) was not detected in any of the experimental plots, whereas eicosapentaenoic acid (C20:5n3) of heterotrophically cultured C. vulgaris (C4-3) containing a doubled concentration of nitrogen was measured to be 1.4 μg mg-1 which was the highest value measured out of all. Arachidonic acid (C20:4n6) of autotrophically cultured C. vulgaris (C4-3) was 1.2-1.3 μg mg-1, but it was not detected at all in Chlorella sp. (C4-8). The amount of n-3 highly unsaturated fatty acid (HUFA) was much higher with autotrophic culture in all three species (p < 0.05). When cultured autotrophically, n-3 HUFA was found to be the highest in C. vulgaris (C4-3) with 22 μg mg-1, but when cultured heterotrophically, Chlorella sp. (C4-8) showed the highest content of n-3 HUFA with 8-10 μg mg-1. The concentration of the other HUFA such as n-6 and n-9 HUFA of the three species tended to be higher with heterotrophic culture, but no noticeable difference was observed with different concentrations of nitrogen.
Total lipid of C. vulgaris (C4-3) was significantly lower in heterotrophic culture, but the nitrogen concentration of medium did not cause the significant difference (p < 0.05).
The culture method and medium did not have any noticeable impact on the total lipid of C. vulgaris (C4-4). The total lipid of Chlorella sp. (C4-8) was determined to be lower in heterotrophic culture with a doubled concentration of nitrogen. Among autotrophically cultured ones, the total lipid was the highest with 263.9 mg g-1 in C. vulgaris (C4-3) in doubled concentration of nitrogen. And among heterotrophically cultured ones, it was the highest with 200 mg g-1 in the control JM medium of Chlorella sp. (C4-8).
Monosaccharide such as glucose and metabolic substances such as glycerol and acetate are widely used as a carbon source for heterotrophic culture of microalgae (Lee 2001, De Swaaf et al. 2003a, 2003b, Ip and Chen 2005). A carbon source has an impact on growth and metabolic activity for heterotrophic microalgal species (Yokochi et al. 1998, De Swaaf et al. 2003a). In this study, three species of Chlorella were able to utilize glucose as a carbon source. However, they were incapable of growing in the experiment plot in which glycerol and acetate were
used for culture (Shi et al. 1999, Ip and Chen 2005).
With regard to growth rate, three Chlorella species showed a similar tendency in the experiment on glucose concentration. The growth of cells seemed more accelerated in the earlier stages in the experimental plots in which 1-2% glucose was added, but the growth was much weaker in 4-5% glucose. This tendency seems to be caused by the growth of cells in the early stage that was hindered when glucose concentration was 4% or above (Xiong et al. 2008). Although the growth of the cells with 1-2% glucose slowed down around day 3-4, the glucose concentration with 4-5%, in turn, led to relatively longer growth period compared to 1-2% glucose concentration and it took longer times to reach the exponential phase (Shi et al. 1999). The optimum concentration of glucose varied with cell density and species of Chlorella. But a continuous growth can be expected additional quantity of glucose is added in every 3-4 days after initial culture with 1-2% concentration.
As for the culture temperature, the optimum temperature for C. vulgaris (C4-3) seems to be 28℃ since it grew most rapidly at 28℃, but slowly at 30℃. But that for both C. vulgaris (C4-4) and Chlorella sp. (C4-8) was determined to be 26℃. Although C. vulgaris (C4-3) and Chlorella sp. (C4-8) were unable to grow at 30℃ or above temperature, C. vulgaris (C4-4) grew well at 30℃ and its growth rate remained fairly high at 32℃ compared to that of 24℃. This led to a conclusion that C. vulgaris (C4-4) was most resistant to high temperature. The optimum temperature 26-28℃ for three heterotrophic Chlorella species in this study was consistent with the previous reports (Shi et al. 2002, Wu and Shi 2007, Xiong et al. 2008).
It has been reported that the ratio of nitrogen to phosphorus, along with concentration of nitrogen and phosphorus contained in a medium, has an impact on growth of microalgae and synthesis of lipid and fatty acid (Liu and Lee 2000, Choi et al. 2002, Tripathi et al. 2002, Wen and Chen 2003). In this study, only the concentration of nitrogen and phosphorus was doubled and tripled that of JM medium, respectively, but no significant difference was observed. This can be explained by the fact that the initial amount of glucose with 1% as a carbon source used in this experiment was insufficient for a rapid growth (Ip and Chen 2005).
When the growth of Chlorella species was compared between heterotrophic and autotrophic culture, it was concluded that the growth rate and cell size were significantly higher with heterotrophic culture than autotrophic one (Griffiths 1965). With the heterotrophic culture in this study, the cell density of C. vulgaris (C4-4) and Chlorella sp. (C4-8) reached approximately 2,000 × 104 cells mL-1, whereas the cell density of C. vulgaris (C4-3) was approximately 1,200 × 104 cells mL-1. However, the biomass in dry weight of C. vulgaris (C4-3) was the highest among three species with 0.8 g L-1. It can be explained by the fact that the cell size of this species was 0.5 μm larger than that of the other two species.
As for the fatty acid compositions, the content of 16:0 and n-3 HUFA was dramatically lower when Chlorella was cultured heterotrophically. Although the total lipid of C. vulgaris (C4-3) and Chlorella sp. (C4-8) was lower when heterotrophically cultured, there was not any difference in C. vulgaris (C4-4). This led to a conclusion that the total lipid varied with species. However, in all three species, the concentration of nitrogen did not seem to have much influence on the fatty acid compositions. Even in the same microalgal species, the lipid content of microalgae depends on temperature, nutrient, culturing method, etc. (Ratledge and Wynn 2002, Wen and Chen 2003, Chisti 2007). Thus, further study on chemical composition of microalgae is needed in detail.
As a result of this study, it was concluded that when Chlorella was cultured heterotrophically, the growth rate, cell size, and fatty acid compositions were affected by temperature, glucose concentration, nutrient in medium and strain. In addition, we suggest C. vulgaris (C4-3) among three species to be the most ideal heterotrophic Chlorella species for industrial application since it had a high biomass and lipid content.