Marado is a small rocky island located off the south coast of Jeju Island and acts as the first gateway of the Kuroshio Current to Korean coastal ecosystems. This island is one of the most unpolluted and well preserved sea areas around the Jeju coast. We extensively observed macroalgal assemblages of species and functional forms in the intertidal and subtidal zones through four seasons on Marado, Jeju Island, Korea to demonstrate the seasonality of vertical distribution patterns and biomass. A total of 144 species (14 Chlorophyta, 40 Phaeophyta, and 90 Rhodophyta) were identified in quadrats and were analyzed seasonally and vertically to define the variation patterns. The annual mean biomass of macroalgae was 2,932.3 g wet wt m-2 and the highest value was recorded in spring and the lowest was in winter. The annual dominant species by biomass was Ecklonia cava followed by Sargassum fusiforme, S. macrocarpum, Amphiroa galapagensis, Chondria crassicaulis, and S. thunbergii. Obvious biomass zonation patterns of macroalgal species were detected in relation to tidal height and depth. Macroalgal biomass, diversity index (H'), and community dynamics were the highest in the shallow subtidal zone. Species number was higher in the subtidal than in the intertidal zone and similar throughout the entire subtidal zone. Our results provide revealing insights into the distribution patterns of macroalgal assemblages in an unpolluted sea area around Jeju Island.
Macroalgal ecologists strive to understand the environmental factors and phenomena that affect macroalgal zonation patterns (Choi and Kim 2004, Balata and Piazzi 2008, Konar et al. 2009, Kang et al. 2011). Macroalgal zonation patterns commonly undergo changes in abundance, diversity, and community dynamics through natural processes along tidal height and depth gradients. The highest level of these patterns is generally found at mean low water (MLW) and decreases with both increasing depth and intertidal height (Garrabou et al. 2002, Choi and Kim 2004, Kang and Kim 2004, Balata et al. 2006, Konar et al. 2009). Although numerous studies have described these general zonation patterns, recent studies have suggested that these patterns may not be generalizable across geographic regions because peaks in abundance or diversity are not found consistently in particular depth strata (Balata and Piazzi 2008, Konar et al. 2009, Heo et al. 2011, Kang et al. 2011). Therefore, there is great interest in investigating not only these exceptional patterns but also different zonation patterns in species composition, diversity, abundance, and community dynamics among areas at local scales (Konar et al. 2009, Heo et al. 2011, Kang et al. 2011, Shin et al. 2011).
Macroalgal growth is influenced by multi-factorial in-teractions among abiotic and biotic components, such as tide, light, and water movement (Dawes 1998). Tide and light attenuation are two of the strongest factors that influence vertical macroalgal distribution (Grahm et al. 2009). Tide is related to the period of desiccation for macroalgal species living in the intertidal zone, where exposure occurs for significantly longer periods in the upper region than in lower areas (Doty 1946). Macroalgal zonation of the intertidal zone is affected by different photosynthetic recovery capacity after water stress (Davison and Pearson 1996, Ji and Tanaka 2002). The vertical distribution of subtidal macroalgae is mainly controlled by the light gradient which is changed depending on the water depth or turbidity (Irving and Connell 2002, Balata et al. 2006). Light attenuation shows a geometric progression curve and only 15% of sea surface radiation reaches a 40 m depth in the clearest of ocean (Littler et al. 1985, 1986). The compensation irradiance (
Littler and Littler (1984) divided various seaweeds into six functional form (F-form) groups based on their morphology, texture, photosynthetic rate, and growth strategy. According to Steneck and Dethier (1994), algal biomass and functional group diversity are highest in habitats with high productivity potential or low disturbance potential dominated by leathery and corticated functional forms. In contrast, habitats with low algal biomass and functional group diversity are dominated by crustose algae having high disturbance potential. Orfanidis et al. (2001, 2003) developed the ecological evaluation index (EEI) to evaluate ecological status based on the characteristic tolerance of each F-form group.
Macroalgal assemblage patterns along the coast of Jeju Island, Korea were initially presented by Lee and Lee (1976). They investigated vertical macroalgal assemblage patterns in the intertidal zone as well as exposure frequency and documented the characteristics of species that were distributed at each vertical level. Although Jeju Island has received considerable attention since the 1970s by phycologists interested in floristic and taxonomic aspects, few ecological studies have examined the abundance, diversity, and community dynamics of macroalgae. Only two spatiotemporally comprehensive studies have been conducted to examine the seasonality of macroalgae in the intertidal / subtidal zone of Jeju Island (Yoo 2003, Kang et al. 2011).
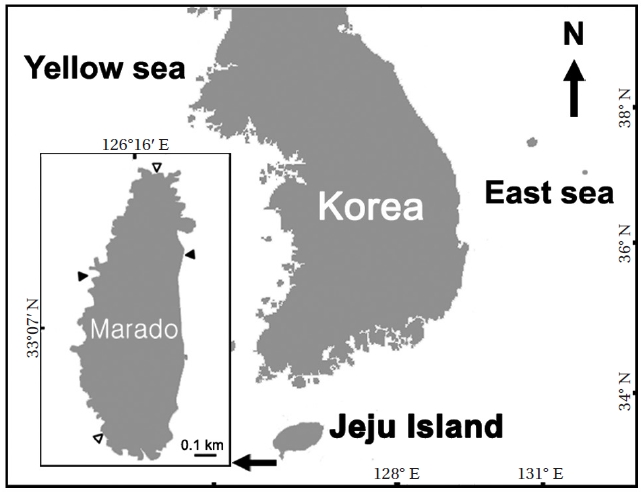
Marado is a small rocky island located off the south coast of Jeju Island, the southernmost part of the Korean coast. It acts as the first gateway of the Kuroshio Current to Korean coastal ecosystems. This area is important both geopolitically and in terms of marine ecology, but the study of macroalagal assemblage patterns around Marado has been lacking.
The present study describes the characteristics of the vertical macroalgal assemblages on the Marado coast and provides baseline data necessary for long-term monitoring. The specific objectives were to examine seasonal variations in vertical assemblage patterns and biomass of macroalgal functional forms in an unpolluted sea area. This is the first study that has intensively examined macroalgal assemblages in the intertidal and subtidal zones of Marado.
Marado is located 8 km south of Jeju Island (Fig. 1). It is elliptical, measuring 0.5 km along its smaller east-west axis and 1.3 km across its north-south axis. The eastern and western coasts are composed of vertical cliffs, but thenorthern and southern areas are gently sloped. The intertidalsubstrate consists mainly of basaltic bedrock, butthe eastern and western parts of the subtidal zone have accumulated rocks and boulders.
Macroalgal assemblages were examined seasonallyfrom June 2010 to May 2011. Three quadrats (0.25 m2)were randomly placed at each of the high-, mid-, andlow-intertidal levels in the southern and northern parts of the island. Nine quadrats were placed at intervals of 2 mdepth in the subtidal zone, at each site in the eastern andwestern parts (Fig. 1). These sites were selected becausethe eastern and southern coasts cannot be approachedby land due to the presence of vertical cliffs, and thenorthern and southern coasts have strong tidal currents that hamper underwater investigations. We divided theareas into three subtidal levels (0-6, 7-12, and 13-18 mdeep from the MLW) to examine variations in macroalgal assemblage with seawater depth. Therefore, three quadratswere placed at each vertical level for each season. Allmacroalgae, except melobesioidean algae, were collected in the quadrats, and the biomass of each macroalgalspecies was measured (g wet wt m-2) in the laboratory. Samples were identified using a microscope based on the descriptions of Lee (2008) and Yoshida (1998). We divided all macroalgae into six functional forms using the criteria of Orfanidis et al. (2001) and compared each percentage among the vertical levels. We calculated diversity (
and dominant (
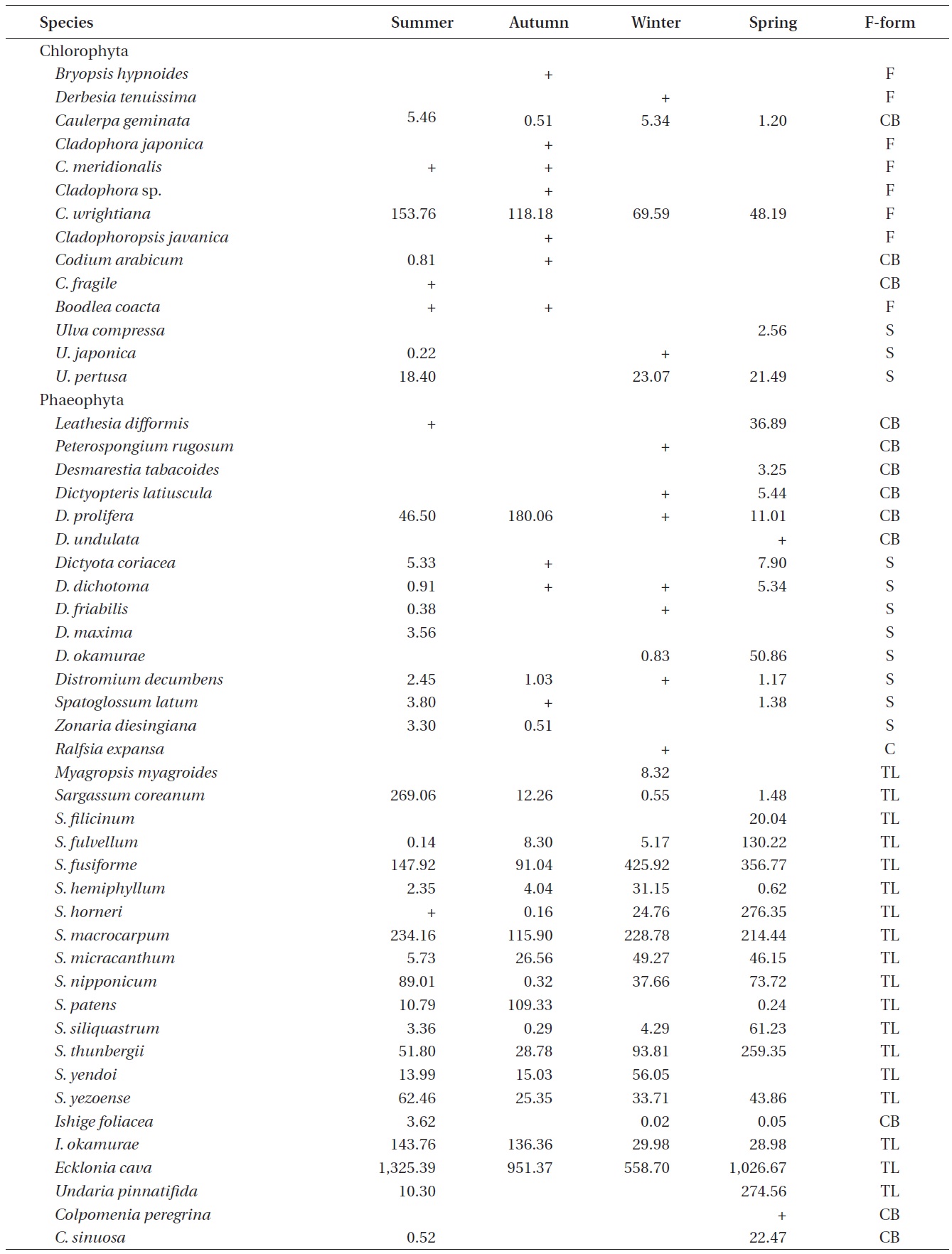
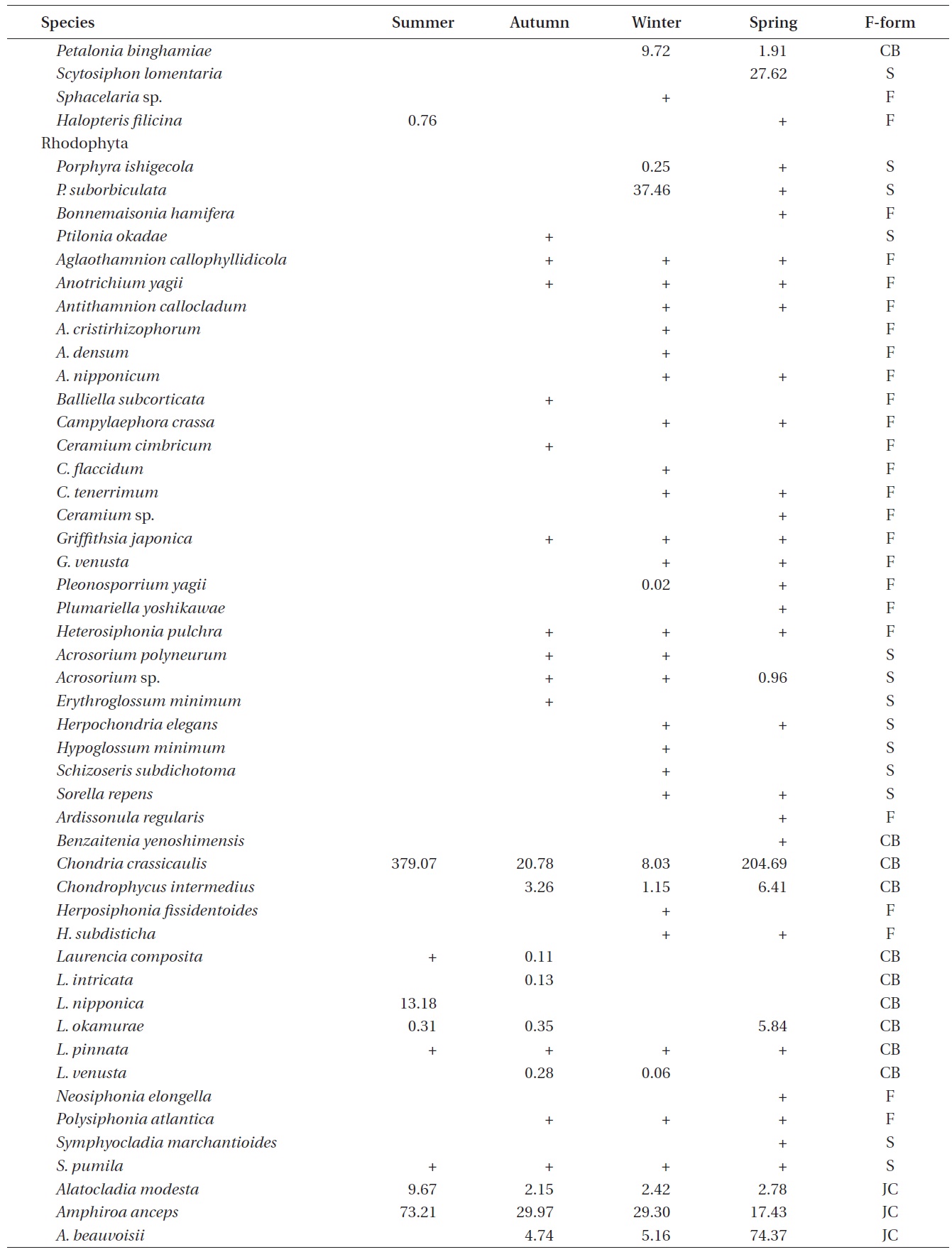
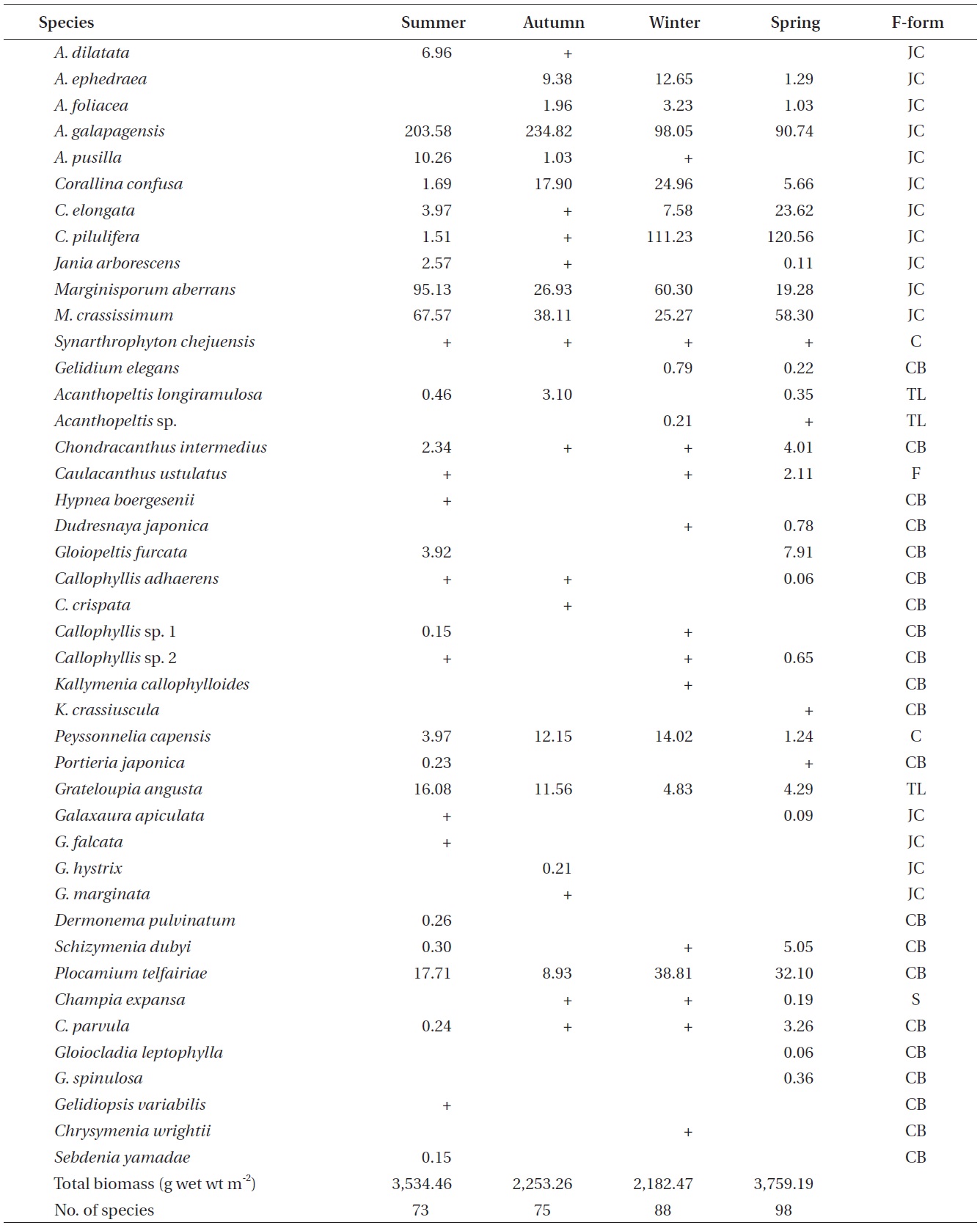
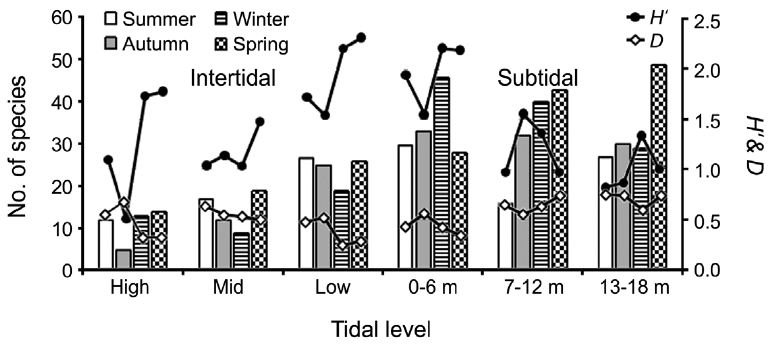
A total of 144 macroalgal species, including 14 Chlorophyta, 40 Phaeophyta, and 90 Rhodophyta, were identified during the study period. Species richness was maximum in the spring and minimum in the summer (Table 1). The annual dominant species by biomass (g wet wt m-2) was
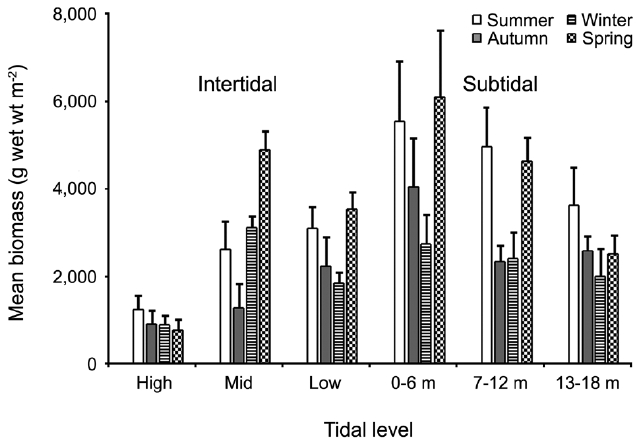
The biomass of dominant seaweeds showed seasonal and vertical variations (Fig. 2). Obvious zonation patterns were observed in the biomass of macroalgal species in relation to tidal level and depth. The annual dominant species at each of the six vertical levels were
The annual mean biomass (g wet wt m-2) of macroalgae was 2,932.3; the highest value was recorded in spring (3,759.2) and the lowest was observed in winter (2,182.5). Fluctuations in vertical macroalgal biomass by season are shown in Fig. 3. Biomass was generally the greatest in the shallow subtidal zone and decreased with both increasing depth and intertidal height. However, biomass was higher in the mid-intertidal zone than in the low-intertidal zone during the winter and spring. This exceptional pattern was due to abrupt increases in the abundance of
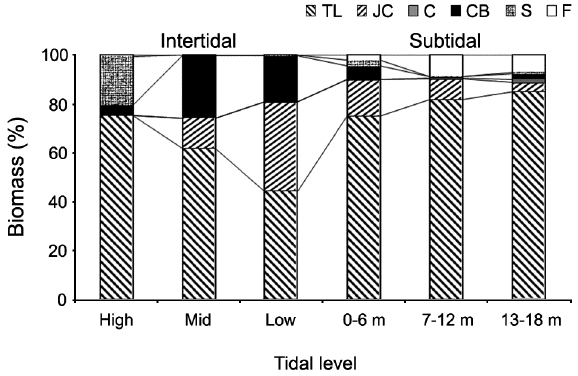
Vertical variations in percent biomass of the six functional seaweed groups are shown in Fig. 4. Thick-leathery group algae (TL) were the most abundant (70.63%), followed by jointed calcareous (JC, 12.70%), coarsely branched (CB, 9.53%), filamentous (F, 2.95%), sheeted (S, 3.90%), and crustose (C, 0.27%) form algae. The abundance percentage of the TL group was the lowest (44.44%) in the low intertidal level and increased toward both the higher intertidal and deeper subtidal sides. The JC group algae showed an opposite trend to the TL group algae. CB group algae were most abundant in the mid-intertidal level and decreased gradually toward the lower vertical level. S group algae appeared sporadically but were most abundant in the high-intertidal level. The F group algae were the highest in the subtidal level at depths of 7-12 m and then decreased toward both the upper and lower levels. Algae in the C group were rare throughout the vertical levels.
Seasonal and vertical variations in species number, diversity indices (
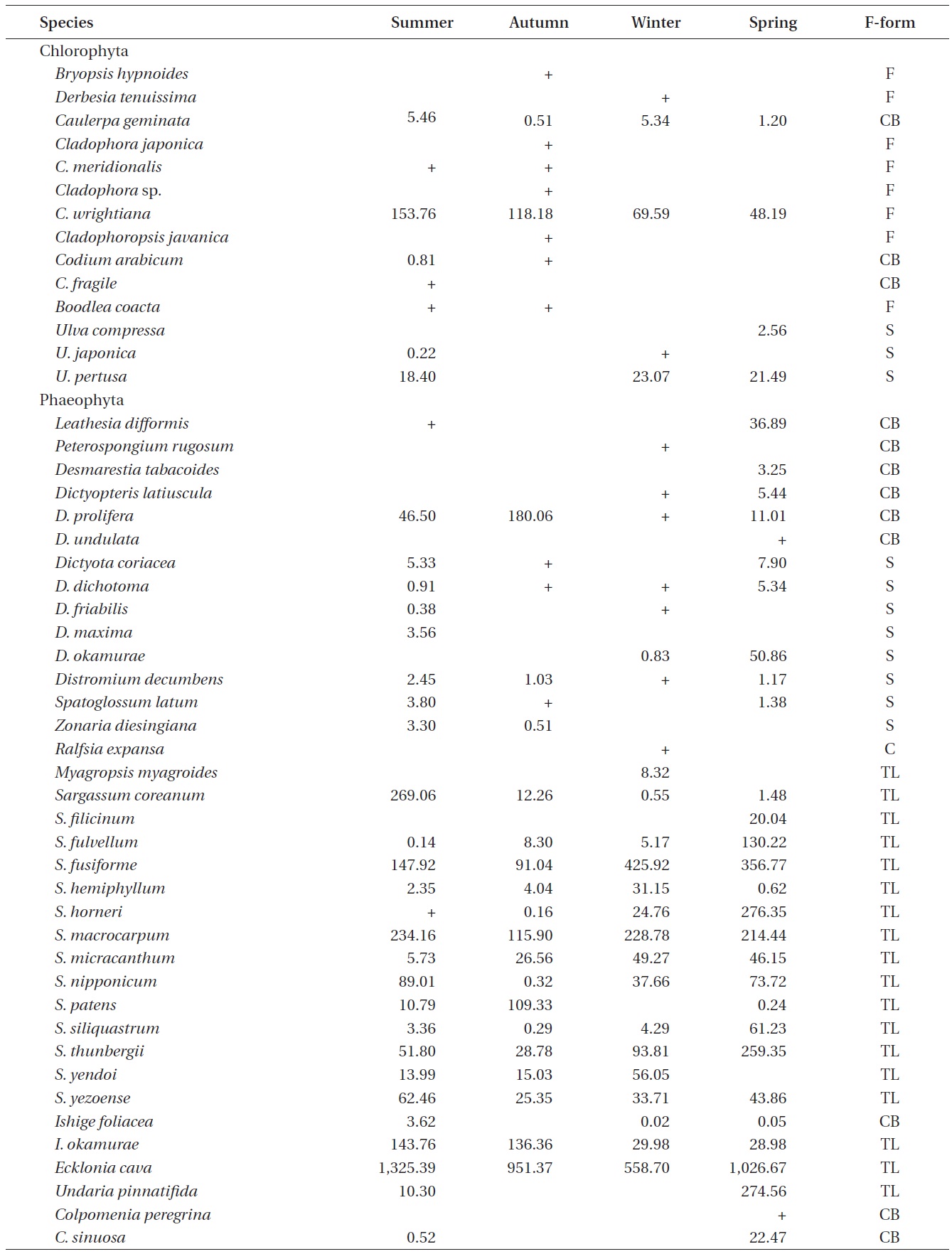
Seasonal variations in macroalgal species biomass (g wet wt m-2) from Marado, Jeju Island, Korea, June 2010 to May 2011
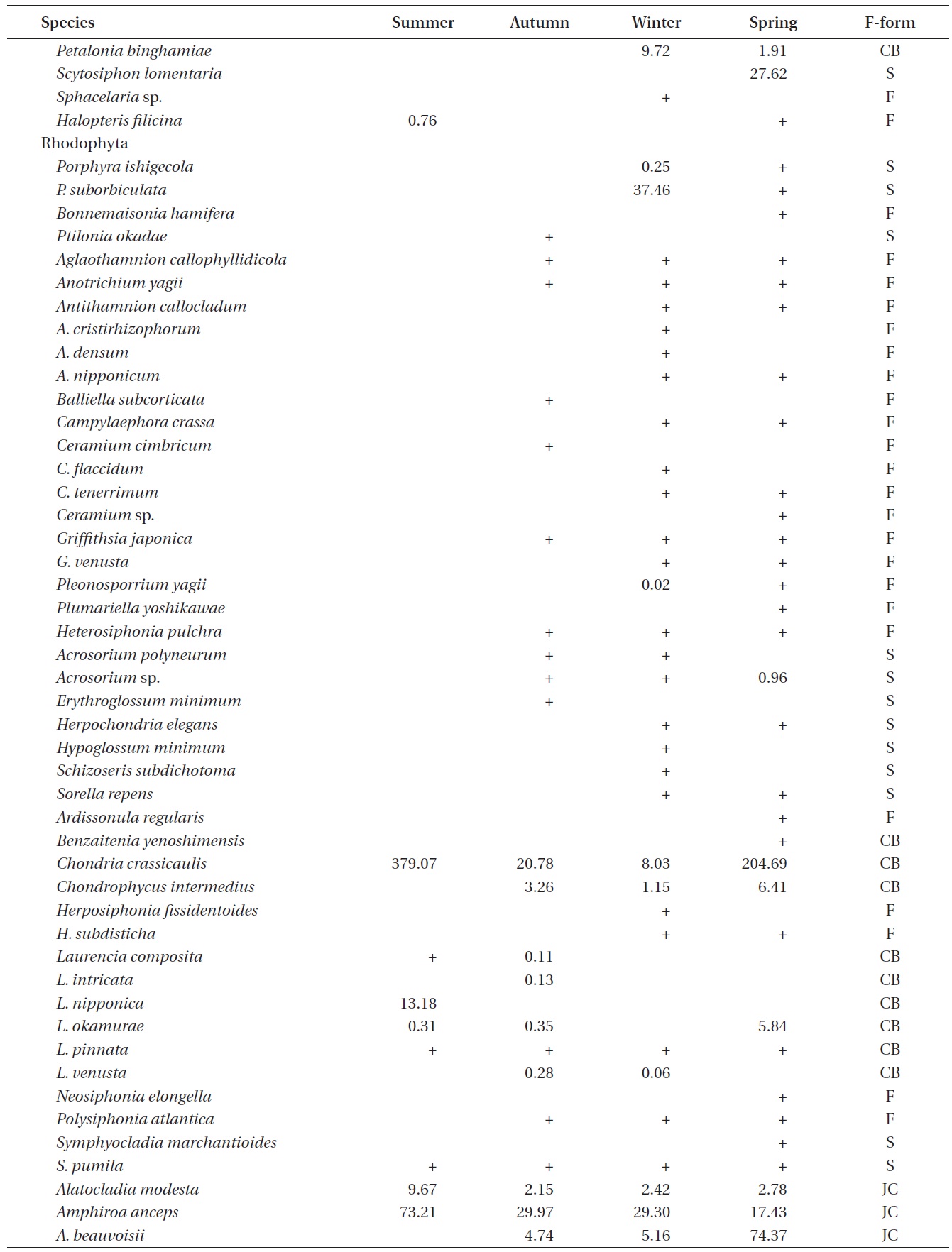
Continued
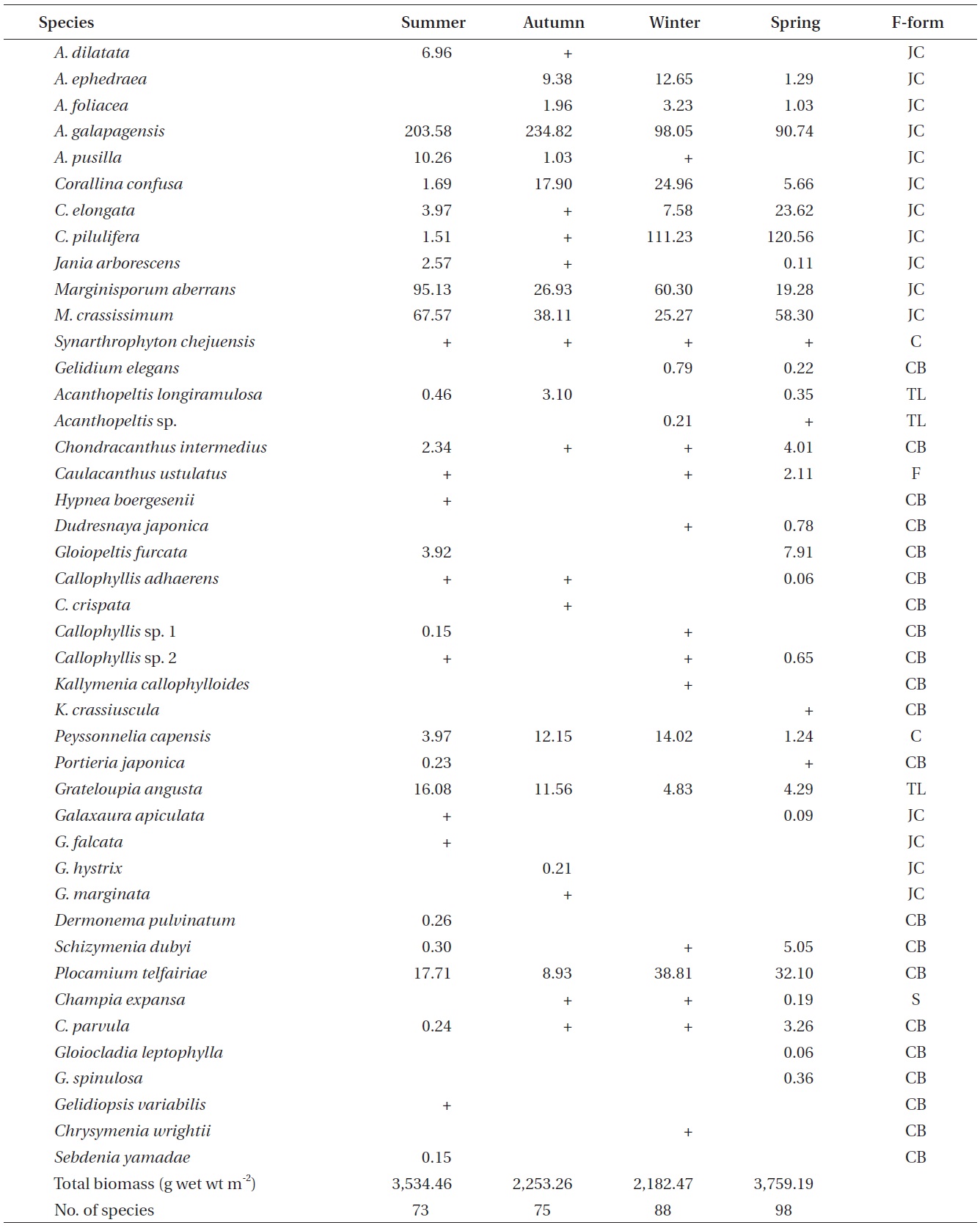
Continued
creased toward the lower tidal level in the intertidal zone. However, species number in the subtidal zone was very similar among vertical levels annually (66 at 0-6 m; 68 at 7-12 m; 69 at 13-18 m depth). No seasonal or vertical trends were observed.
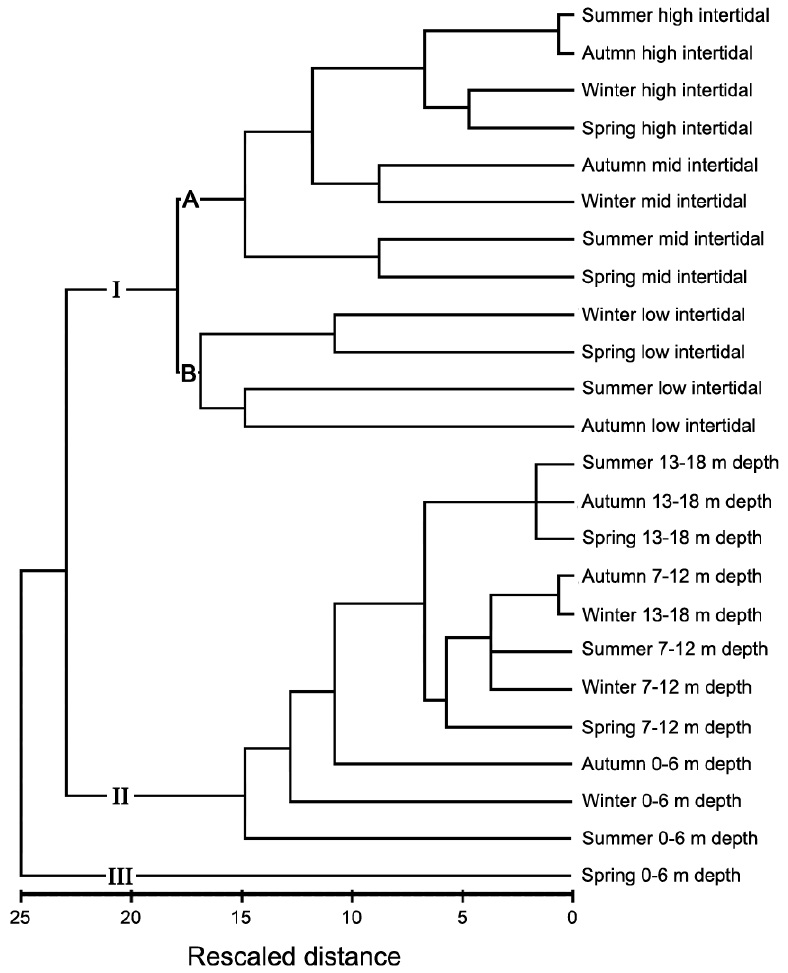
Fig. 6 presents a cluster analysis dendrogram based on
similarities in macroalgal species abundances. The data were clearly divided into three groups representing the intertidal zone (I), most of the subtidal zone (II), and the shallow subtidal (0-6 m depth level) in spring (III). The trends in similarity distances varied with position on the shore: there were weak trends observed around MLW (low intertidal and 0-6 m depth levels). Group I was be divided into subgroups A and B. Subgroup A commonly contained relatively higher biomass levels of
Lee and Lee (1982) documented the dominant species distributed along each vertical level in the intertidal zone of Jeju Island through seasonal investigations. Species included
Kang et al. (2011) reported that macroalgae in the high and mid-levels of the intertidal zone are dominated by desiccation-resistant species, whereas deeper subtidal zones are dominated by kelp with a wider surface area. In addition, dominant species near the sea surface (low intertidal to shallow subtidal zone) vary with the degree of wave action. Their results correspond well with the results of our study (Figs 2 & 3). Zonation patterns in macroalgal community composition (Fig. 2) in the intertidal zone seemed to be strongly influenced by desiccation stress toward the upper extreme and by wave stress toward the lower extreme. We observed communities of dried
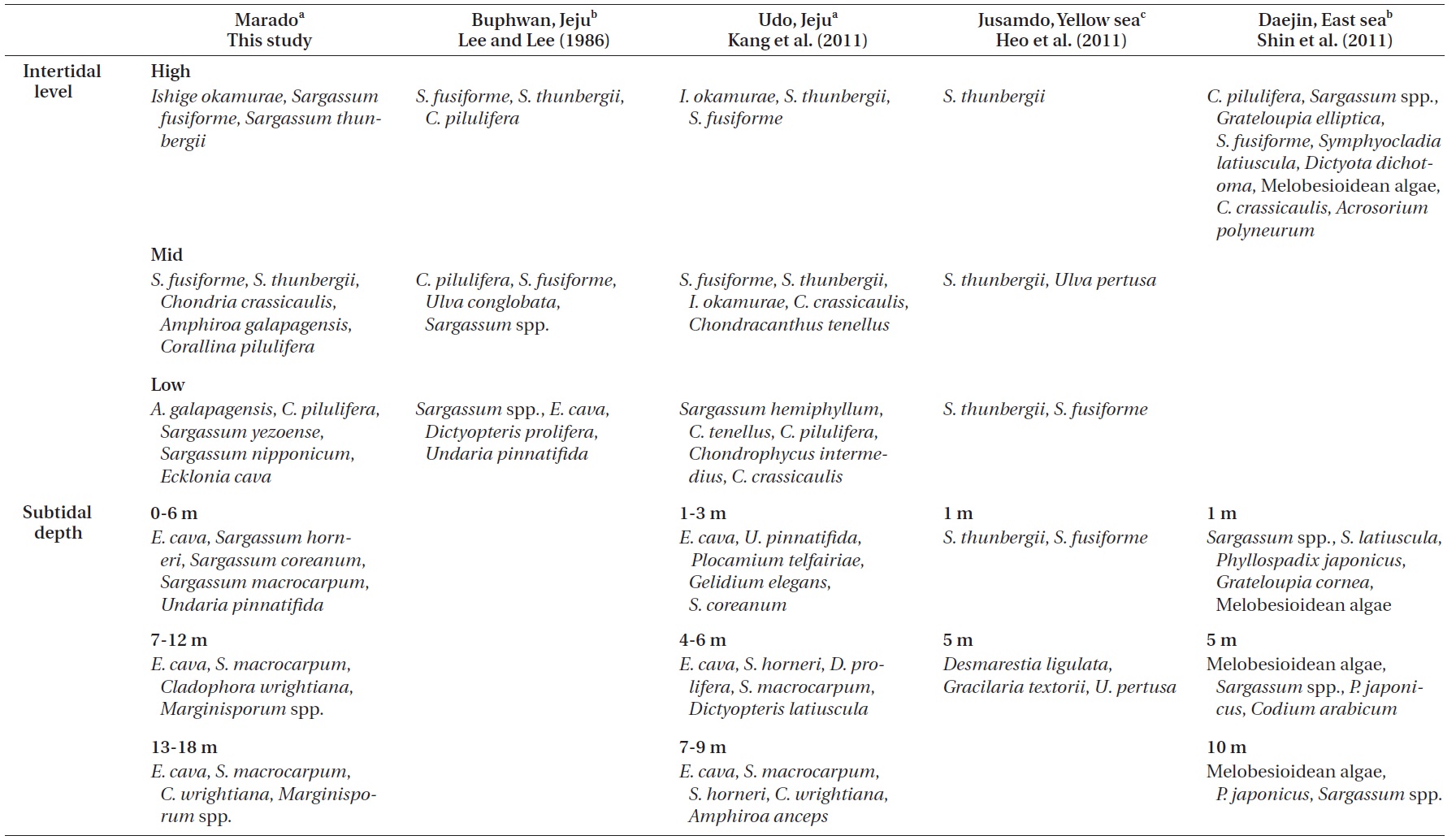
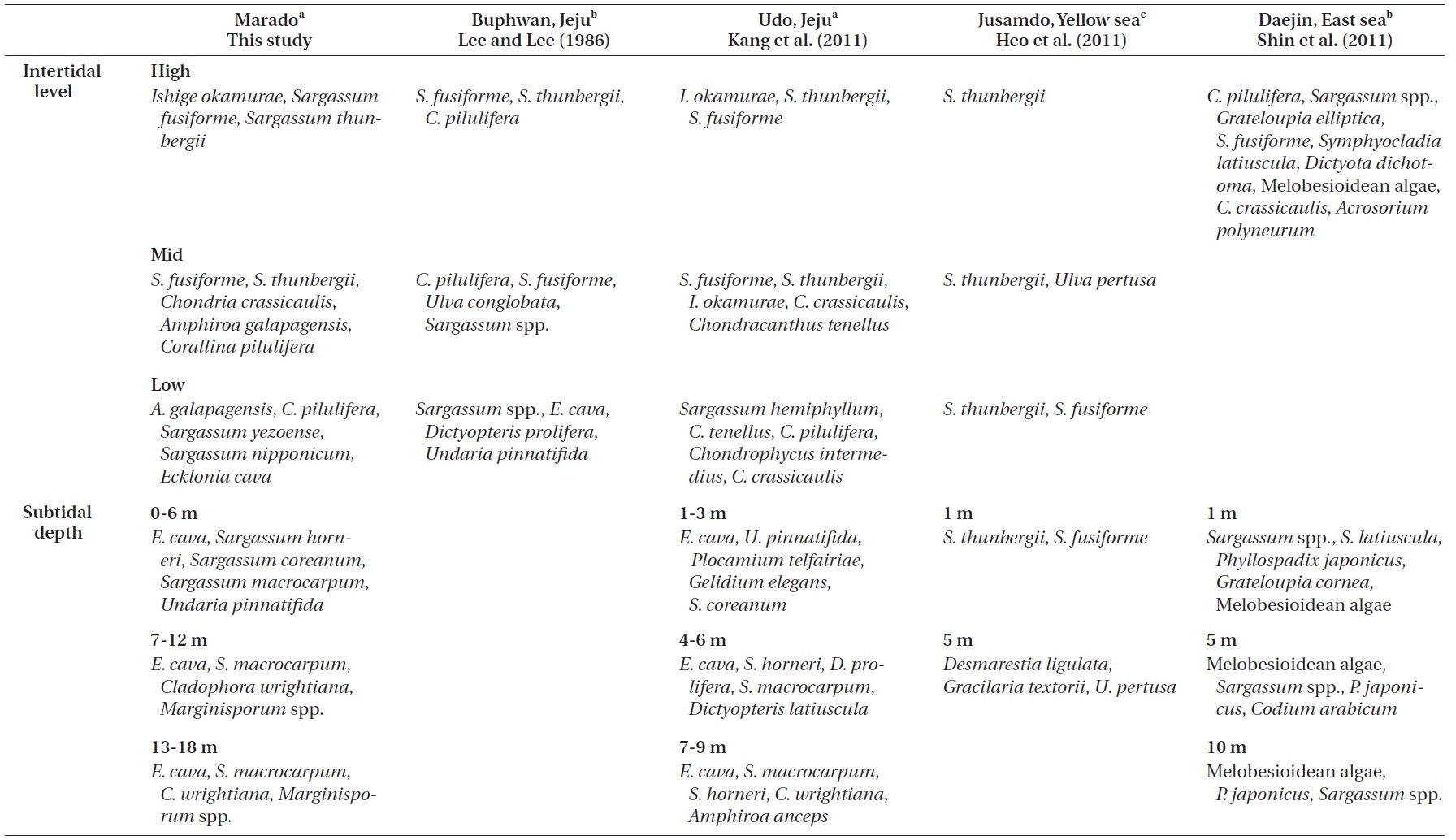
Comparisons of the vertical distribution patterns of representative macroalgal communities among this and previous studies
comprised of finger-like holdfasts, a single solid stipe, and large leathery blades. These morphological distribution patterns in the subtidal zone seem to be the result of the water motion gradient (Stewart and Carpenter 2003, Fowler-Walker et al. 2006). The ratio of filamentous algae increased toward the deep water caused by
The general patterns of abundance, diversity, and community dynamic in the macroalgal assemblages among shore levels and along depth gradients are represented by an increase from the high intertidal zone seaward and a decrease with depth in the subtidal zone (Garrabou et al. 2002, Balata et al. 2006, Konar et al. 2009). Similarly, we found that macroagal biomass and diversity index values (
In summary, our intensive study of macroalgal assemblages on the Marado coast provided great insights into the distribution pattern of macroalgal assemblages in unpolluted marine areas of Jeju Island. Our results also suggested that water motion in addition to tides and light penetration gradients is the major factor affecting the vertical distribution pattern in macroalgal assemblages. This information on macroalgal assemblage patterns from an uncontaminated marine area provides vital evidence for establishing effective management strategies to restore macroalgae by selecting area, season, and depth.