Eels are catadromous species that hatch in the sea but migrate as elvers (pre-juveniles) to freshwater to grow to adulthood. Artificial eel-spawning and even aquaculture of the complete life cycle have been conducted, and some experimental efforts have been successful (Ohta and Tanaka, 1997; Ohta et al., 1997; Kim et al., 2007; Kagawa et al., 2009). However, the supply of elvers for aquaculture still depends on natural resources which is the principal restriction on the volume of eel culture. In addition, elver arrest, especially in
We previously reported that the major nutrients (vitamins A and E, cholesterol, and fatty acid composition) of
Eels
>
Analysis of general compounds
The proximate composition of general compounds (water content, crude protein, crude lipids, and ash) contained in cultured eels was analyzed using Association of Official Analytical Chemists methods (1995). Water and fat contents were measured using a CEM automatic extractor (Labwave 9000/ FAS 9001; CEM Corp., Matthews, NC, USA). Protein was analyzed using a Kjeltec System (Kjeltec Auto 2400/2460; Foss Tecator AB, Hoganas, Sweden), and ash was measured using an ash analyzer (Mas 7000; CEM Corp.). In addition, the proximate composition of various adult eel FFs that were on sale in October 2011 were also determined using the above methods.
For analyses of vitamins A (VA) and E (VE), 0.5 g freezedried sample, 3 mL 95% ethanol (EtOH), and 0.5 m 10% pyrogallol/EtOH were added to a test tube and mixed well. Then 1 mL 50% KOH solution was added to the tube, and the sample was saponified in a 95℃ water bath for 30 min and cooled rapidly on ice. Then we mixed in 3 mL distilled water and petroleum ether (v/v), after which only the petroleum ether layer was collected. This step was repeated three times. The resulting solution was filtered using anhydrous Na2SO4 to remove moisture and then evaporated using a rotary evaporator. The concentrate was dissolved in 1 mL isopropyl alcohol for VA analysis or in 1 mL hexane for VE. After filtration through a 0.2 μm membrane filter, the solution underwent HPLC analysis for vitamin content using an HPLC column (μ-Porasil, 4.6 × 250 nm; Waters, Milford, MA, USA) and nhexane: isopropanol (99.92:0.08, v/v, isocratic mode) as the mobile phase. The flow rate was 1 mL/min. Detection was conducted at 336 nm for VA and at 280 nm for VE, using a UV/ VIS detector (Model 2489; Waters).
One gram of freeze-dried sample and 3 mL 95% EtOH were added to a test tube and extracted using sonication for 30 min. Subsequently, 1 mL 50% KOHA was added to the tube, and the solution was incubated in a 50℃ water bath for 1 h and then cooled rapidly on ice. Next, 5 mL hexane was added to the solution, and after mixing for 5 min, 1 mL of the upper layer (organic layer) was removed and evaporated to dryness using N2 gas. Then we added 3 mL Liebermann-Burchard reagent, and the sample was vortexed and placed at room temperature for 30 min. Then the absorbance was measured at
Extractions for fatty acid analysis followed the procedure of Folch et al. (1957), and the extract was methylated according to Morrison and Smith’s method (1964). The supernatant of methylated extract was removed and stored at -80℃. The analysis was conducted using a gas chromatograph equipped with an autoinjector (Varian 3400; Varian, Oshawa, ON, Canada). The instrumental conditions are described in Cho et al. (2011).
All measurements were performed in triplicate. The results of all measurements are expressed as means ± SD. If necessary, results were analyzed using one-way ANOVA with a 95% confidence level.
We reported previously that the major nutritional ingredients in cultured eels significantly varied across eel farms (Cho et al., 2011). To determine the mechanism driving this result, we must first examine the extent to which differences in FFs or in physical/chemical conditions among farms affect the main nutritional content of eels. Five different eel farms, each using just one of four FFs from different companies to feed mature eels, were selected for analyzing the levels of VA, VE, cholesterol, and fatty acids in mature eels (about 300-350 g) at each farm. In addition, for comparative evaluation among FFs under the same environmental conditions, 15 1-ton tanks were installed at Farm A (in Yeonggwang-gun, Jeollanam-do, Korea), and the above ingredients were analyzed after feeding eels (25-30 g) each FF for 6 weeks.
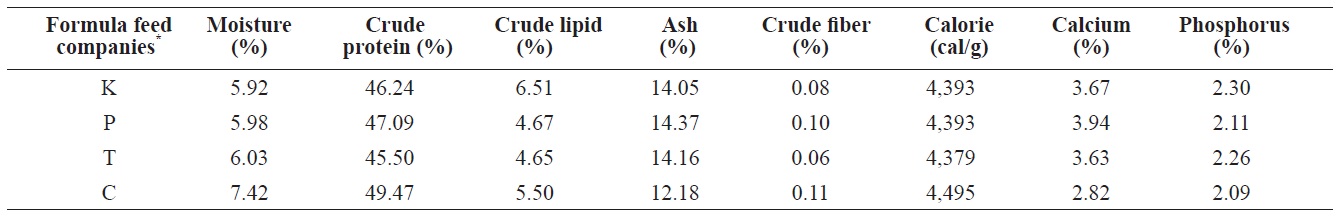
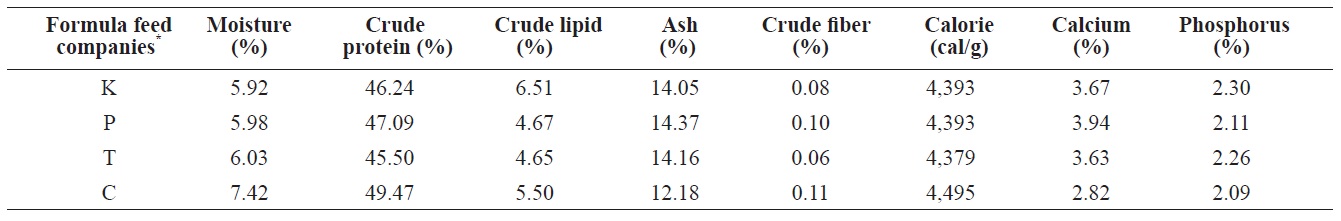
The proximate composition of nutrients (i.e., water content, crude protein, crude lipids, ash, calcium, phosphorous, and crude fiber) must be determined to register FFs for fish in Korea (Statute Book for Feed Management, 2002). The contents of several main nutritional ingredients ((VA, VE, and fatty acids) of eels were determined for eels fed the four different
The comparison of proximate compositions between four different formula feeds for mature eel culture
types of FFs (Tables 1 and 2).
The proximate composition of adult eel FFs was also determined (Table 1). Moisture content ranged from 5.92% (K Co. FF) to 7.43% (C Co. FF). Crude protein levels, a main factor determining both eel growth and the feed efficiency of an FF, did not significantly differ across the four FFs, ranging from 45.5% (T Co. FF) to 49.47% (C Co. FF). The protein requirements of eels depend on size and species, but protein content must be approximately 45-50% (Arai et al., 1971; Nose and Arai, 1973; Degani et al., 1985 Han et al., 2005). Crude lipids, which contain essential fatty acids needed for the normal growth and survival of fish, ranged from 5.5% (C Co. FF) to
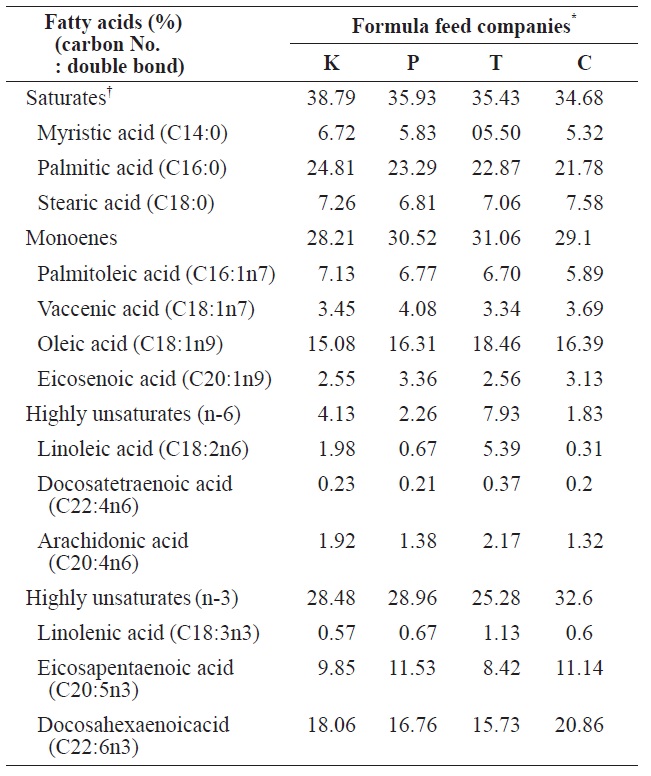
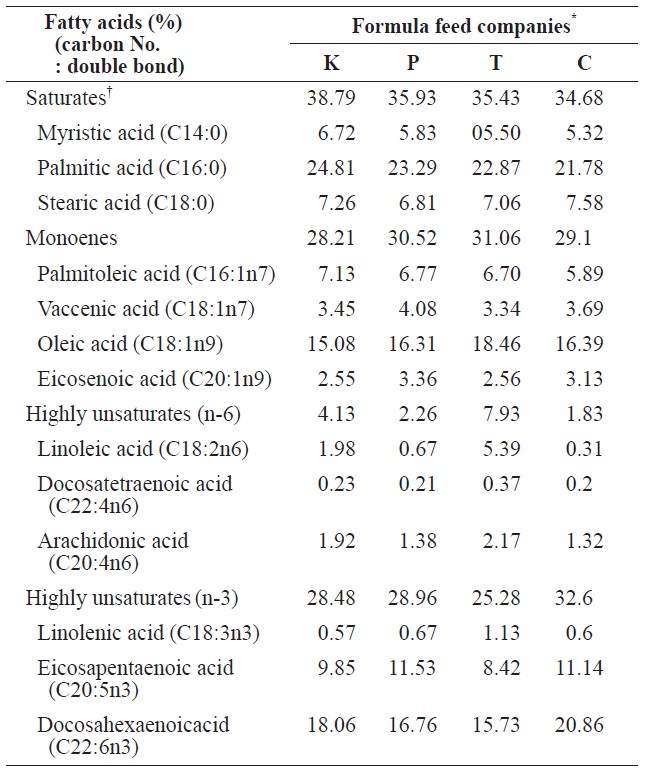
The comparison of fatty acids compositions between four different formula feeds for mature eel culture
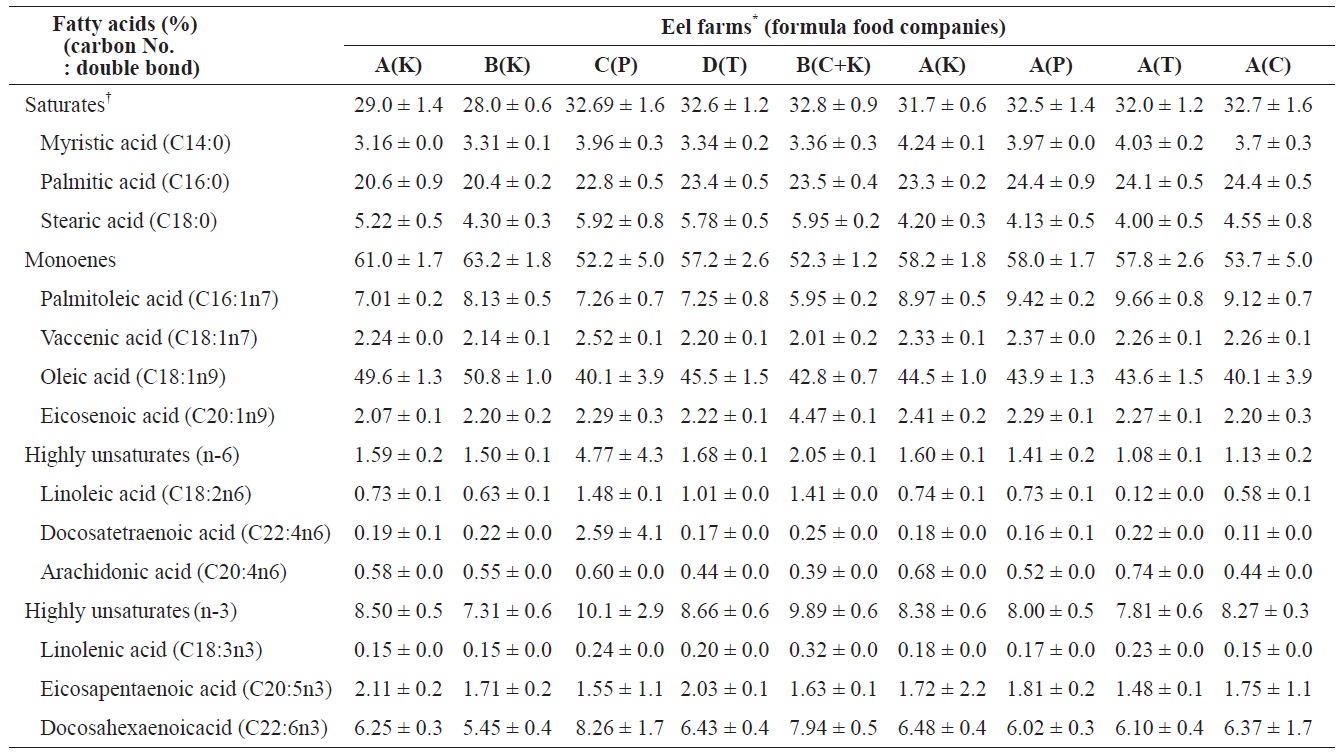
6.51% (K Co. FF). Ash and crude fiber contents ranged from 0.11% (C Co. FF) to 14.37% (P Co. FF) and from 0.08% (K Co. FF) to 12.18% (C Co. FF), respectively, and these variables did not significantly differ among the tested FFs. Calories ranged from 4,379 cal/g (T Co. FF) to 4495 cal/g (C Co. FF), calcium content ranged from 2.82% (C Co. FF) to 3.67% (K Co. FF), and phosphorous content ranged from 2.11% (C Co. FF) to 2.3% (K Co. FF). Because the fatty acid composition of FFs has been shown to affect the growth and fatty acid composition of cultured eels (Takeuchi et al., 1980; Bae et al., 2004), the fatty acid composition of the four FFs was also analyzed. Saturated fatty acids (SFA) and monounsaturated fatty acids (MUFA) did not differ among the four FFs. In contrast, the highly unsaturated fatty acid (HUFA) ω-6 HUFA ranged from 1.83% (C Co. FF) to 7.93% (T Co. FF), and ω-3 HUFA levels were highest in C Co. FF (32.6%) and lowest in T Co. FF (25.28%). These differences may have been caused by differences in the type of fish meal or oil used as feed additives.
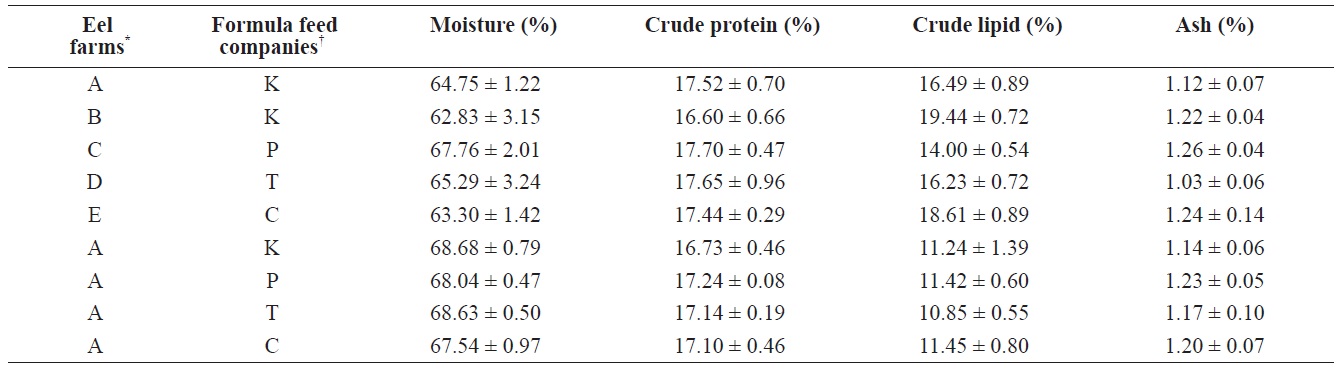
For the eels fed the four different FFs at the same eel farm, the proximate composition analysis revealed no significant differences in moisture (67.5 ± 1.0% to 68.7 ± 0.8%), crude protein (16.7 ± 0.5% to 17.2 ± 0.1%), crude lipids (10.9 ± 0.6% to 11.5 ± 0.8%), or ash (1.1 ± 0.1% to 1.2 ± 0.1%), suggesting that although the proximate composition did vary somewhat among FFs and between domestic and imported FFs, these differences were not large enough to affect the quality or proximate composition of cultured eels. In contrast, the results from eels cultured at the five different farms were markedly different. Across the five farms, moisture content of the eels ranged from 62.8 ± 3.2% (Farm B, K Co. FF) to 67.8 ± 2.0% (Farm C, P Co. FF), but values did not significantly differ among farms. Crude protein levels were lower in eels from Farm B (16.6 ± 0.7%), but the values from the other farms were similar (about 17.5%). Crude lipids in cultured eels significantly differed across farms; eels from Farm B had the highest crude lipid content (K Co. FF, 19.4 ± 0.7%), whereas
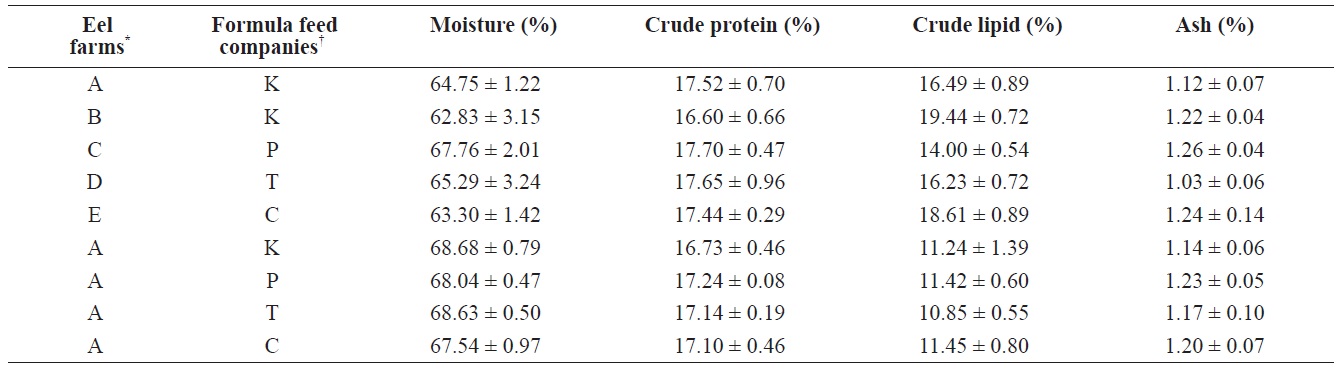
The comparison of proximate compositions between eels fed with four different formula feeds for mature eels and between eels cultured at 5 different eel farms where eels fed with one of the four different formula feeds
those from Farm C had the lowest crude lipid content (P Co. FF, 14.0 ± 0.5%). Ash content did not significantly differ across either the four FFs or the five farms (Table 3). Because the difference between domestic FF brands was not significant, the effect on the proximate composition of eels was not expected to be significant. However, because the proximate compositions of eels from farms that used different FF brands did significantly differ, differences in the physical or chemical environments of each farm likely strongly affected the major proximate composition of cultured eels.
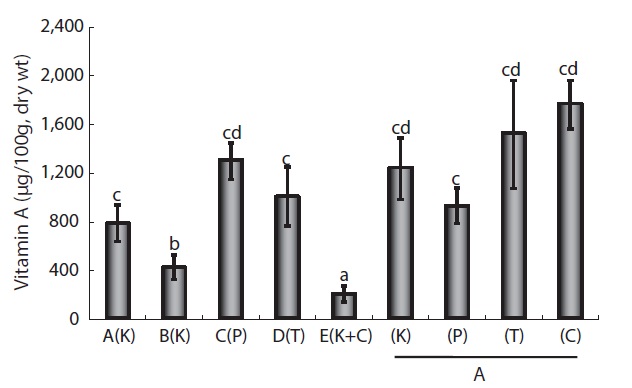
The VA content of wild mature eels is typically 1 mg/100 g dry weight (wt; about 3,500 IU), although values vary depending on the species and tissue analyzed. In addition, VA levels tend to increase with the age and size of fish (Edisbury et al., 1937; Cho et al., 2006). VA content differed among the four FFs, but values were very low compared to those in eels (data not shown). VA content differed among eels fed the four different FFs; values were highest in those fed C Co. FF (1.76 ± 0.3 mg/100 g dry wt) and lowest in those fed P Co. FF (0.92 ± 0.25 mg/100 g dry wt), indicating that VA content may be somewhat correlated with the content in FFs. Across the five farms that used different FFs, differences in VA content were more remarkable, being highest (1.3 ± 0.25 mg/100 g dry wt) in eels from Farm C (P Co. FF) and lowest (0.42 ± 0.2 mg/100 g dry wt) in those from Farm E (T Co. FF) (Fig. 1). The differences in eel VA content among FFs and among farms were not significant, suggesting that differences in culture techniques or culture environments more strongly affect the VA content of eels compared to variation in VA content of different FFs.
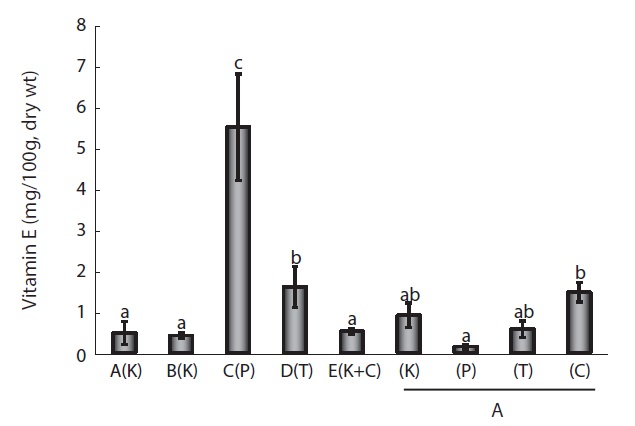
VE (α-tocopherol) content was compared in the same samples as described above for VA (Fig. 2). Tocopherol in cultured eels is composed of primarily the α-type and small amounts of the β- and γ-types (Cho et al. 2011). VE levels in wild eels are 4-7 mg/100 g dry wt, and the content of cultured eel muscle in Japan is 21-37 mg/100 g dry wt, which serves as an indicator of the quality of cultured eel (Furuita et al., 2009). Compared
to eels fed each of the four FFs, those fed the C Co. product had the highest VE levels (1.47 ± 0.5 mg/100 g dry wt), whereas those fed the P Co. product had the lowest (0.15 ± 0.1 mg/100 g dry wt); however, overall values were not high compared to the content in cultured eels. In eels cultured at the five different farms, those from Farm C had the highest content (5.5 ± 2.3 mg/100 g dry wt), while eels from the other farms ranged from 1.6 ± 1.0 to 0.42 ± 0.2 mg/100 g dry wt. Based on
these results, differences in VE levels among commercial FFs appeared to affect the VE content of cultured eels. Moreover, the environmental differences in cultures across farms also affected VE content. In summary, the fat-soluble VA and VE contents in fresh eel varied depending on the commercial FF used. Vitamin levels were much more strongly affected by the culture environment, including differences in water purity, the types of microorganisms present, and whether vitamin supplements were administered.
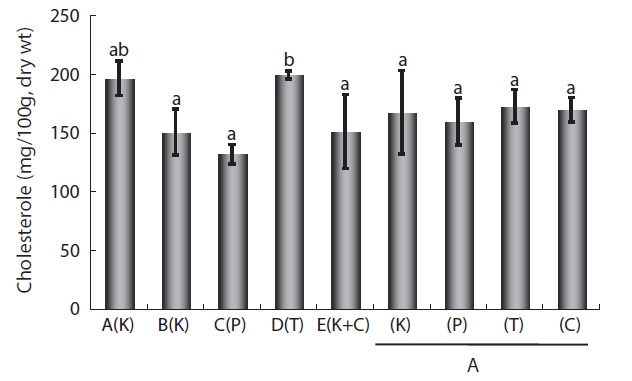
In an experimental system, Burgos et al. (1990) demonstrated that cholesterol, a major nutritive component of eels, constituted nearly 7.5% of total lipids in the liver and about 1% in the muscle of control eels. Cholesterol content in fish was proportional to the amount of lipids, and lipid content was affected by various environmental (diet, season, water temperature) and biological factors (age, sex, size). However, these factors do not necessarily lead to significant changes in protein and ash contents (Jeong et al., 1998). Thus, we determined whether cholesterol content varied across cultured eels fed different FFs and among eels cultured at different eel farms. For eels fed one of the four FFs under the same culture conditions (i.e., at the same farm), cholesterol content ranged from 172 ± 10 mg/100 g dry wt to 159 ± 3 mg/100 g dry wt and did not significantly differ among FF types, which was consistent with the lack of a significant difference in the crudelipid content of eels fed the different FFs (Table 2). However,similar to the results for VA and VE contents, cholesterol content significantly differed for eels cultured at the five different farms the highest content was 198.6 3.4 mg/100 g dry wt at Farm A (K Co. FF), and the lowest content was 131.4 6.8 mg/100 g dry wt at Farm C (P Co. FF) (Fig. 3), these differences did not appear to be correlated with the type of FF and were likely related to the farm itself, i.e., the eel culture environment. Although the cholesterol content and fattyacid content of eels differed across the five different farms, the lower levels of cholesterol and fatty acids in Farm C eels are likely related to each other.
The SFA, MUFA, and HUFA of cultured eel flesh/whole body eel were compared (Table 4). SFA and MUFA did not significantly differ across eels fed different FFs, whereas -6HUFA did significantly vary among FFs. Values of ω-6 HUFA in eels fed K Co., P Co., C Co., and T Co. FFs were 1.60 0.1%, 1.41 ± 0.2%, 1.13 ± 0.2%, and 1.08 ± 0.1%, respectively, whereas the values of ω-3 HUFA in eels at the same farms/ FFs were 8.38 ± 0.6%, 8.0 ± 0.52%, 8.27 ± 0.29%, and 7.81 ± 0.55%; however, ω-3 HUFA did not significantly differ across farms (
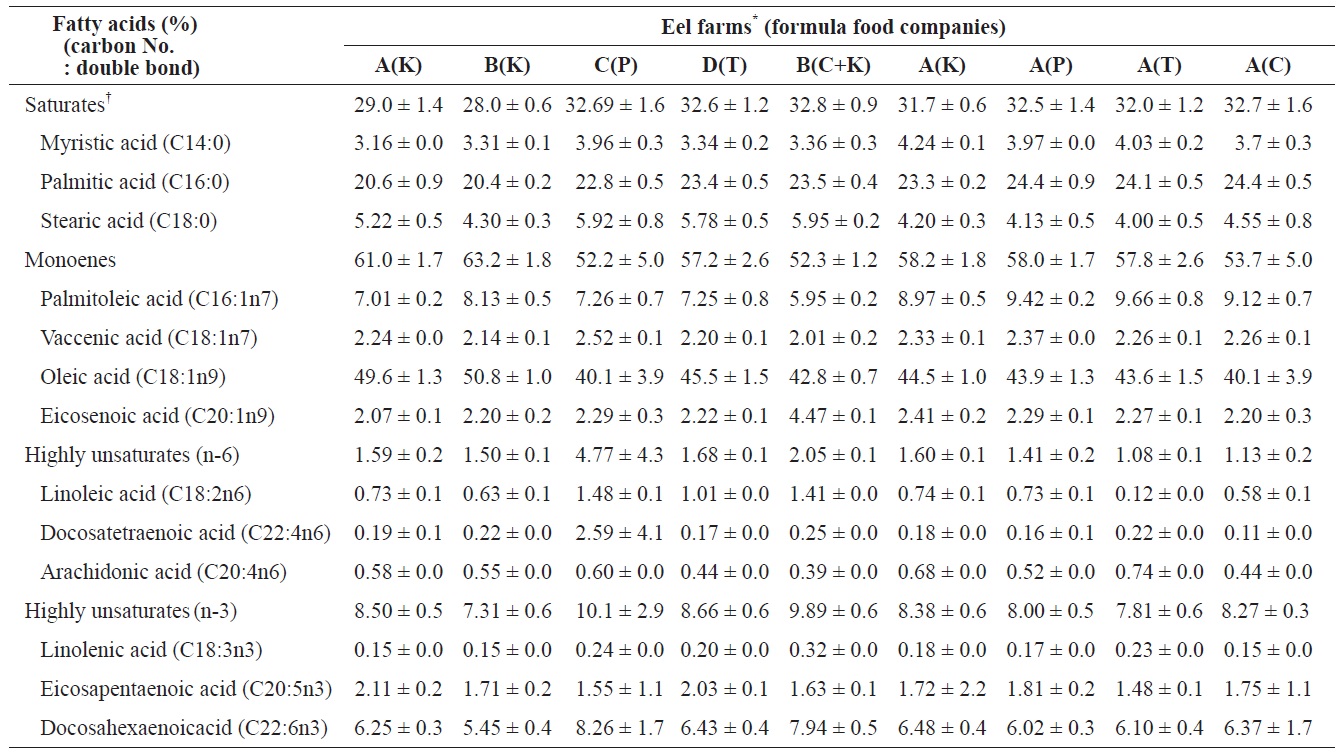
The fatty acids compositions in total lipids of eels fed with four different formula feeds for mature eels and between eels cultured at the five different eel farms where eels fed with one of the four different formula feeds
linolenic acid (n-3) in eel food did affect the composition of fatty acids. However, as shown in the present study (Tables 2 and 4), the differences in HUFA content among FFs did not significantly affect the composition of fatty acids in mid-sized eel cultures. In fact, previous studies have reported that the composition of fatty acids is also affected by lack of food or non-nutritional factors including water temperature (Watanabe et al., 1983; Satoh et al., 1984; Bell et al., 1986).
Similar to VA, VE, and cholesterol, the levels of some fatty acids significantly varied across eels cultured at different farms. SFA ranged from 32.7 ± 1.6% (Farm C, P Co. FF) and 32.6 ± 1.2% (Farm D, S Co. FF) to 29.0 ± 1.4% (Farm A, K Co. FF) and 28.0 ± 0.6% (Farm B, K Co. FF). MUFA was highest at Farm B (63.2 ± 1.8%) and lowest at Farm C (52 ± 5.0%) and significantly differed across eels cultured at different farms. ω-6 HUFA and ω-3 HUFA also significantly varied among farms, with the highest values at Farm C (4.77 ± 4.3% and 10.1 ± 2.9%, respectively) and the lowest values at Farm B (1.5 ± 0.1% and 7.31 ± 0.6%, respectively). Of the ω-3 HUFAs, EPA ranged from highest to lowest at Farm A (2.11 ± 0.2%), Farm D (2.03 ± 0.1%), Farm B (1.71 ± 0.2%), and Farm C (1.55 ± 1.1%), whereas DHA exhibited values in the following order: Farm C (8.26 ± 1.7%), Farm D (6.43 ± 0.4%), Farm A (6.25 ± 0.3%), and Farm B (5.45 ± 0.4%). The composition of fatty acids in cultured eels did not significantly differ across farms, in contrast to previous reports by Choi et al. (1985) (SFA, 30.5%; MUFA, 53.6%; HUFA, 8.0%) and Cho et al. (2011) (SFA, 30.5 ± 1.3% to 33.0 ± 0.5%; MUFA, 59.9 ± 2.0 to 55.8 ± 0.5%; HUFA, 8.0 ± 0.5% to 9.2 ± 0.7%). The difference in the composition of fatty acids among FFs was not as high as expected, which may account for the small difference in quality between domestic and imported FFs. On the other hand, because fatty acid composition differs somewhat between eels cultured in a still-water system” anda water-recirculation system (Cho et al., 2011), our results also suggest that environmental differences experienced by cultured eels at different farms could more strongly affect thefatty acid composition than differences among eels fed differentFFs. In particular, Farm C exhibited higher values of ω-6 and -3 HUFA compared to the other eel farms, suggesting that the culture conditions at Farm C have certain advantages. An operator of Farm C mentioned that they use special methods based on their experiences, but these methods have not been empirically proven and thus require more detailed experimental study.
In conclusion, eel-culture techniques still must be standardized and systematized to produce high-quality eels. In particular, while eel quality did not significantly differ between eels fed domestic or imported FFs and because domestic FFs cannot be considered superior to Japanese eel FFs, domestic FFs must be improved. In addition, because significant differences in eel quality were observed among eel farms, it is necessary to determine the specific environmental factors that affect eel quality. Further studies focused on identifying these factors and applying culture techniques for high-quality eel culture are needed.