Eighteen specimens of juvenile Mugilidae were collected in October 2012 from the southern coastal waters of Jeju Island, and identified based on analysis of their mitochondrial DNA16S rRNA sequences. Seventeen specimens of Oedalechilus labiosus and a single specimen of Ellochelon vaigiensis were found, constituting a new record for these species among Korean ichthyofauna. O. labiosus is identified by the angle at the posterior end of its mouth, which contains a round notch, a darkish dorsal margin of the pectoral fin, the presence of 33-36 lateral line scales, and 23-24 vertebrae. E. vaigiensis is identified by dark dorsal and pectoral fins, the presence of 26 lateral line scales, and 25 vertebrae. The proposed Korean name for Oedalechilus is ‘Sol-ip-sung-eo-sok’ and that for Ellochelon is ‘Nup-jeok-ggo-ri-sung-eo-sok.’ The proposed Korean names for the species are ‘Sol-ip-sung-eo’ and ‘Nup-jeok-ggo-ri-sung-eo’ for O. labiosus and E. vaigiensis, respectively. We present a key for identification of the Mugilidae family of species from Korea, and include these two newly recorded species.
Various subtropical fish can be found in the waters surrounding Jeju Island, Korea (Kim and Lee, 1994), and numerous fish previously unrecorded in these waters have recently been reported (Kim, 2009). The family Mugilidae (order Mugiliformes) is widely distributed in tropical and temperate waters, with 72 species divided into 17 genera described worldwide (Nelson, 2006), among which 15 species and 7 genera have been reported from Japan (Senou, 2002). Jordan and Starks (1905) were the first to report the presence of
In this study, mugilid juveniles were collected by scoop net in October 2012 from the southern coastal waters of Jeju Island. Among the specimens collected, we identified
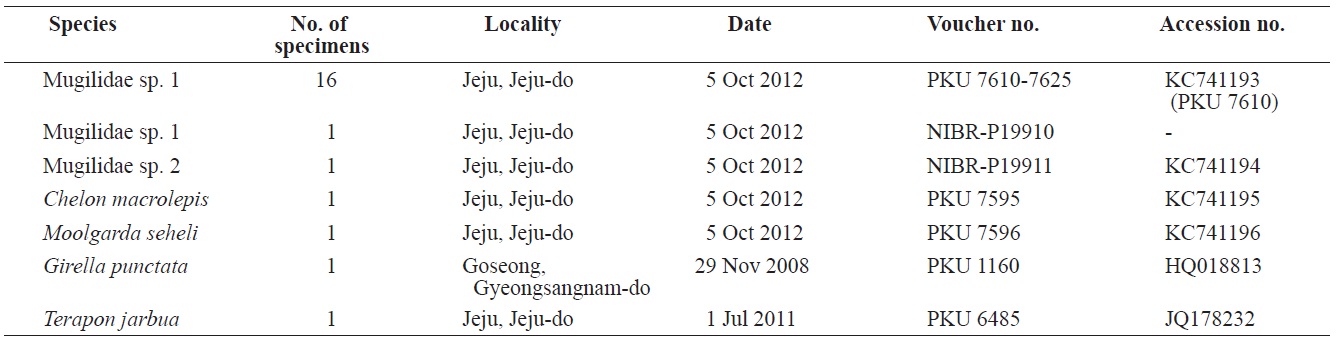
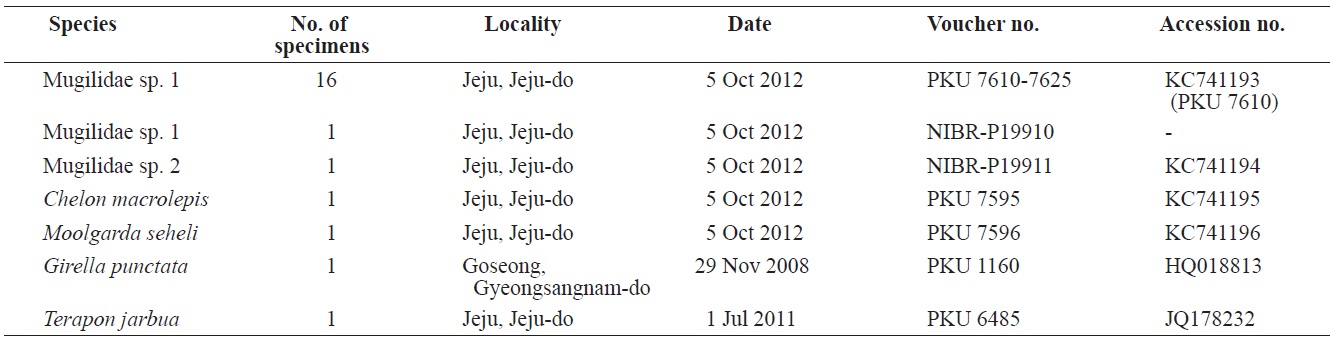
Eighteen specimens of mugilid juvenile were collected from Yerae Port, Seogwipo, Jeju Island, by scoop net in October 2012, and fixed in 99% ethanol as whole fish (Table 1). Counts and measurements were carried out according to Thomson (1997) and recorded to the nearest 0.1 mm using vernier calipers. All fin rays and vertebrae were counted using radiograph images (HA-100; Softex, Tokyo, Japan) and lateral line scales were counted under a stereo microscope (SZX-16; Olympus, Tokyo, Japan). The specimens were kept at the Pukyong National University (PKU) and the National Institute of Biological Resources (NIBR-P), Korea (Table 1).
Genomic DNA extraction and polymerase chain reaction (PCR) for molecular identification were performed according to Kwun and Kim (2010) and Kwun et al. (2012b). Genomic DNA was extracted from muscle tissues using Chelex 100 resin (Bio-Rad, Hercules, CA, USA). PCR was used to amplify the mitochondrial DNA 16S rRNA region using a universal primer set (Palumbi, 1996): 16Sar-L (5′-CGCCTGTTTATCAAAAACAT- 3′), 16Sbr-H (5′-CCGGTCTGAACTCAGATCACGT- 3′). The PCR was conducted using an MJ Mini Thermal Cycler PTC-1148 (Bio-Rad) with the PCR solution containing 10 μL of genomic DNA, 5 μL of 10× PCR buffer, 2.4 μL of 2.5 mM dNTPs, 1 μL of each primer, 0.5 μL of FR-Taq polymerase (BioMedics, Seoul, Korea), and distilled water to bring the final volume to 50 μL. The PCR was carried
[Table 1.] List of specimens of the present study
List of specimens of the present study
out under the following conditions: initial denaturation at 95℃ for 5 min, 35 cycles of denaturation at 95℃ for 1 min, annealing at 50℃ for 1 min, extension at 72℃ for 1 min, and a final extension at 72℃ for 5 min. DNA was sequenced on an ABI 3730XL Sequencer (Applied Biosystems, Foster City, CA, USA) using the ABI PRISM BigDye Terminator v3.1 Ready Reaction Cycle Sequencing Kit (Applied Biosystems). The nucleotide sequences were deposited in the DDBJ/EMBL/ GenBank databases (accession nos. KC741193-KC741194). Sequences were aligned using ClustalW (Thompson et al., 1994) in BioEdit ver. 7 (Hall, 1999), and sequences of
(new Korean name: Sol-ip-sung-eo-sok)
Description
Preorbital containing deep notch and round posterior tip; slender maxilla, slightly curved downward, and with the posterior tip slightly visible when mouth is closed; upper lip thick and broad, lower lip thin; lip with file-like margins; teeth on
vomer and palatines; interorbital region slightly convex; adipose eyelid poorly developed; no spine on the margin of the operculum; pectoral fin length slightly longer than the head length; axillary scale small; caudal fin forked or emarginated; body covered with cycloid scales; 32-52 lateral line scales (Schultz et al., 1953; Thomson, 1997; Harrison and Senou, 1999).
Remarks
Only two species,
>
Oedalechilus labiosus (Valenciennes, 1836) (
(new Korean name: Sol-ip-sung-eo)
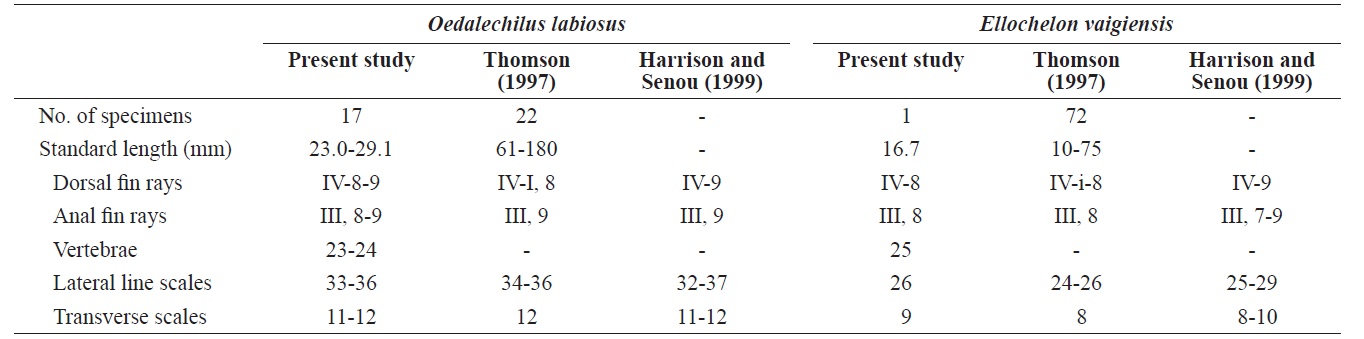
[Table 2.] Comparison of meristic characters of Oedalechilus labiosus and Ellochelon vaigiensis
Comparison of meristic characters of Oedalechilus labiosus and Ellochelon vaigiensis
Material examined
PKU 7610-7625, 16 specimens, 23.0-29.1 mm standard length (SL); NIBR-P19910, 1 specimen, 26.7 mm SL, Yerae Port, Seogwipo, Jeju Island, Scoop net, 5 Oct 2012 (Fig. 1).
Description
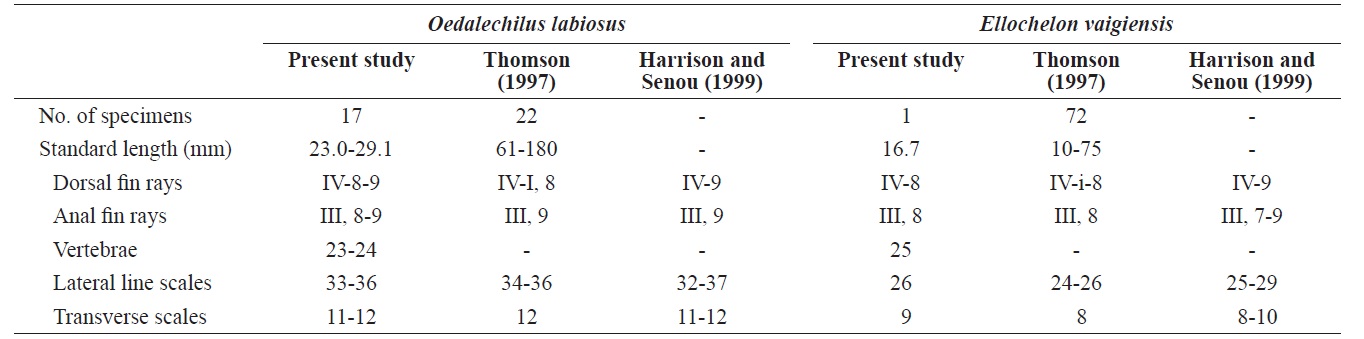
Counts are shown in Table 2. Measurements, expressed as a percentage (mean in parentheses) of the SL, include the following: body depth 24.5-29.2 (27.9); head length 26.6-30.9 (28.9); head width 16.1-19.4 (17.7); snout length 6.5-8.0 (7.2); interorbital width 11.5-15.0 (13.6); eye diameter 9.1-11.2 (10.0); upper lip height 1.7-2.6 (2.2); predorsal length 53.3- 62.3 (57.6); pectoral fin length 20.0-23.3 (22.0); and caudal peduncle depth 11.7-12.8 (12.3).
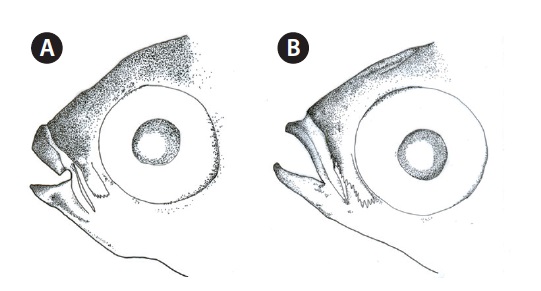
Head broad, and depressed dorsally; body deep and compressed posteriorly; mouth terminal; posterior tip of maxilla beyond the anterior margin of the eye; symphysis blunt; posterior margin of preorbital with 5-6 spines (Fig. 2A); upper lip thicker than lower lip; posterior end of mouth angle with round notch (Fig. 2A); posterior tip of maxilla beyond mouth angle; 1 or 2 rows of curved conical teeth on each jaw; snout short and blunt; interorbital region slightly convex; adipose eyelid absent; 2 dorsal fins completely separated, and origin of 1st dorsal fin located in the middle of the body; posterior tip of pectoral fin extending beyond the vertical line from the origin of the pelvic fin, and posterior tip of the pelvic fin extending beyond the vertical line from the origin of the 1st dorsal fin;
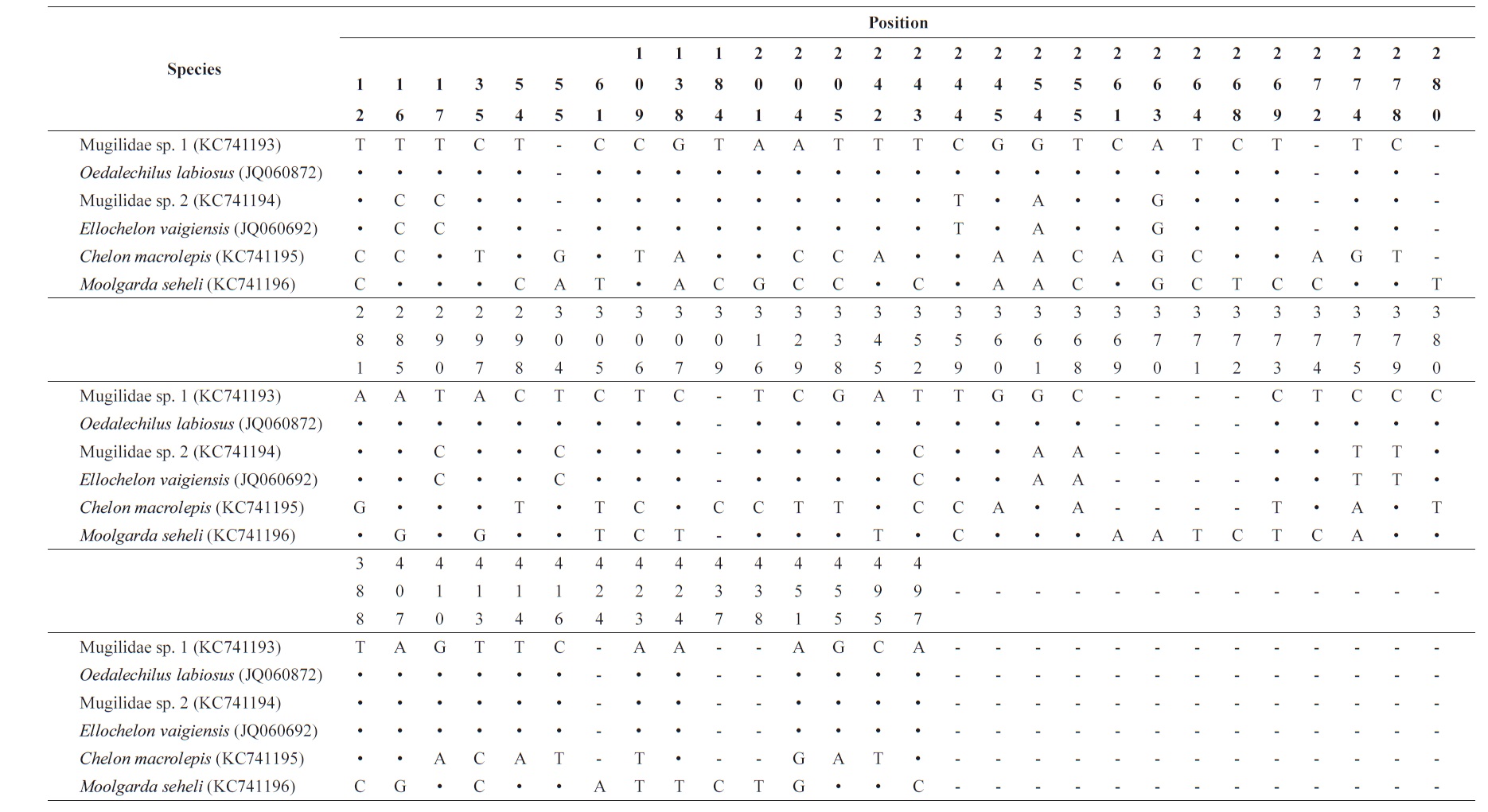
[Table 3.] Nucleotide variable position in consensus sequences of the 16S rRNA
Nucleotide variable position in consensus sequences of the 16S rRNA
origin of the anal fin located in front of the vertical line from the origin of the 2nd dorsal fin; pectoral fin with small axillary scales; caudal fin emarginated; head and body covered with cycloid scales.
Coloration
When fresh, the head and body are silver-whit, darkening dorsally. Both lips are dark. All fins are semitransparent. Tiny melanophores are present on the membranes of the dorsal, caudal, and anterior region of anal fins, with no melanophores on the pelvic fin. Dorsally, the margin of the pectoral fin is dark. After alcohol fixation, the head and body turned a darker silver?white.
Molecular identification
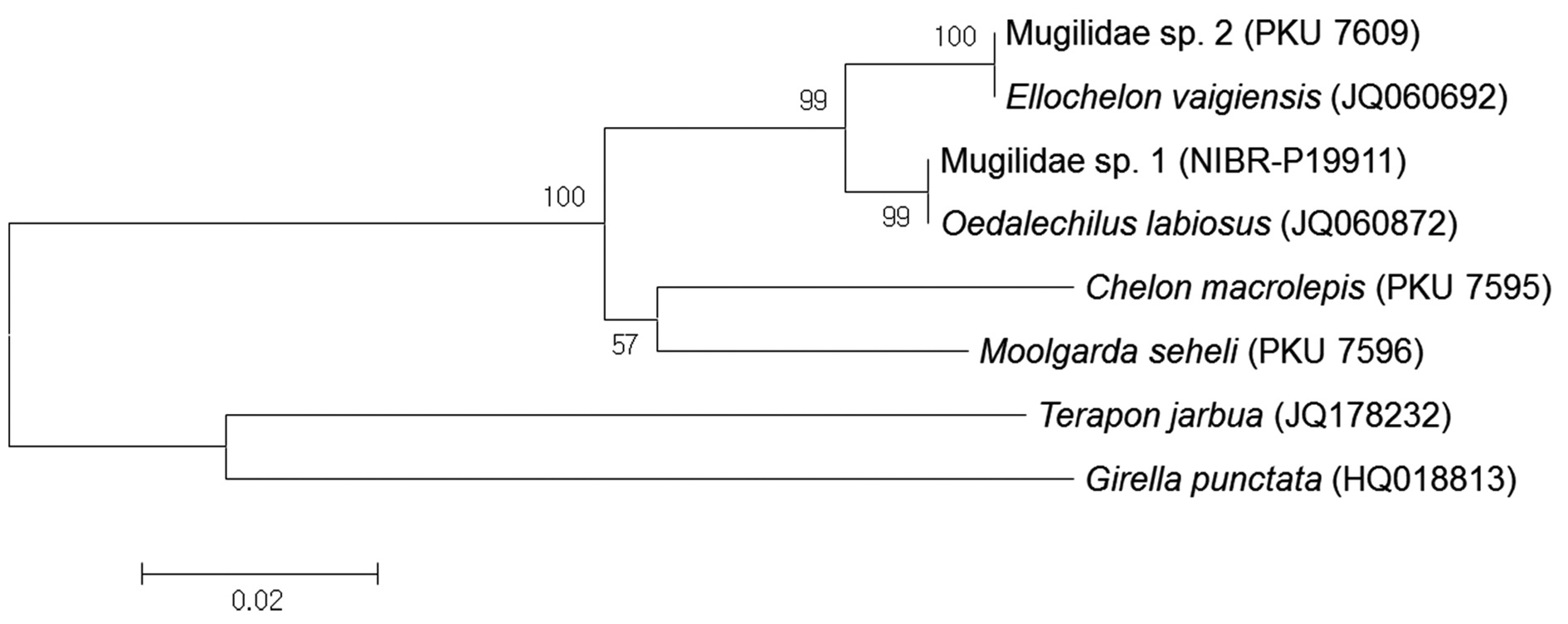
Based on analysis of a 592-base pair (bp) mitochondrial DNA 16S rRNA sequence, Mugilidae sp. 1 corresponds to
Distribution
Remarks
Based on the original description of
(new Korean name: Nup-jeok-ggo-ri-sung-eo-sok)
Description
Posterior tip of preorbital broad and square; maxilla slender, curved downward, and the posterior tip visible when mouth is closed; lips thin; lower margin of upper lip smooth; teeth on vomer and palatines; interorbital region slightly convex; adipose eyelid poorly developed; caudal fin truncated; body covered with ctenoid scales (cycloid in juvenile); 24-29 lateral line scales; pectoral fin black (Thomson, 1997; Harrison and Senou, 1999; Senou, 2002).
Remarks
Only one species,
>
Ellochelon vaigiensis (Quoy and Gaimard, 1825) (
(Korean name: Nup-jeok-ggo-ri-sung-eo)
Material examined
NIBR-P19911, 1 specimen, 16.7 mm SL, Yerae Port, Seogwipo, Jeju Island, Scoop net, 5 Oct 2012 (Fig. 3).
Description
Counts are shown in Table 2. Measurements, expressed as a percentage of the SL, include: body depth 28.7; head length 32.9; head width 19.2; snout length 8.4; interorbital width 15.6; eye diameter 10.8; upper lip height 1.8; predorsal length 58.7; pectoral fin length 12.6; and caudal peduncle depth 18.0.
Head broad and depressed dorsally; body deep and compressed posteriorly; mouth terminal, with posterior tip of the maxilla reaching to the anterior margin of the eye; symphysis slightly pointed; posteroventral margin of the preorbital with numerous spines (Fig. 2B); both lips thin; posterior tip of the maxilla beyond the mouth angle; 1-2 rows of curved conical teeth on each jaw; snout short and slightly pointed; interorbital region slightly convex; adipose eyelid absent; 2 dorsal fins completely separated, and origin of the 1st dorsal fin located behind the midline of the body; posterior tip of the pectoral fin beyond a vertical line at the origin of the pelvic fin, and posterior tip of pelvic fin beyond a vertical line at the origin of the 1st dorsal fin; origin of anal fin located in front of a vertical line at the origin of the 2nd dorsal fin; pectoral fin without axillary scales; caudal fin truncated; head and body covered with cycloid scales.
Coloration
When fresh, the head and body are silver-white and darkish yellow dorsally. Both lips are dark. The dorsal and pectoral fins are black, and the pelvic, anal, and caudal fins are semitransparent. The pelvic and anal fins are yellowish; the caudal fin is light yellow, with tiny melanophores on the membrane. After alcohol fixation, the head and body were darkish silverwhite; and the yellow color of the pelvic, anal, and caudal fins graded to colorless.
Molecular identification
Based on analysis of a 592-bp mitochondrial DNA 16S rRNA sequence, Mugilidae sp. 2 corresponded to
Distribution
Remarks
Based on the original description of
>
Key to the species of family Mugilidae from Korea
1a. Posterior end of mouth angle with round notch. Margin of upper lip with file-like except during early life stage. ········· Sol-ip-sung-eo (new Korean name) Oedalechilus labiosus
1b. Posterior end of mouth angle without round notch. margin of upper lip smooth. ······························································· 2
2a. Pectoral fin black. Caudal fin truncate. ·································································· Nup-jeok-ggo-ri-sung-eo Ellochelon vaigiensis
2b. Pectoral fin light or dark gray. Caudal fin emarginate or forked. ·································································· 3
3a. Posterior end of maxilla reaching to mouth angle. ··········································································· Sung-eo Mugil cephalus
3b. Posterior end of maxilla beyond mouth angle. ··············· 4
4a. Posterior margin of scales on middle of body with membrane. When mouth closed, posterior tip of maxilla not exposed or slightly visible. ················································································ Cho-seung-ggo-ri-sung-eo Moolgarda seheli
4b. Posterior margin of scales on middle of body without membrane. When mouth closed, posterior tip of maxilla exposed.················································································ 5
5a. Keel in front of dorsal fin. .················································································ Deung-jul-sung-eo Chelon affinis
5b. No keel in front of dorsal fin. ·············································· 6
6a. Lateral line scales 30-34. ···················································································· Keun-bi-neul-sung-eo Chelon macrolepis
6b. Lateral line scales 38-44. ····························································································· Ga-sung-eo Chelon haematocheilus