This study examines the optimal fractionation method and conditions for the isolation of endoprotease- and exopeptidase-active fractions from crude extracts of cuttlefish hepatopancreas (HP) using four fractionation methods: ammonium sulfate fractionation (ASF), polyethylene glycol fractionation (PGF), ion exchange chromatography (IEC), and gel filtration chromatography (GFC). Total endoprotease activity highest in the fraction II (concentrate of fractions 34-42; 842.60 U) of GFC, followed by fraction III (40-60% ammonium sulfate fraction; 670.25 U) of ASF, fraction I (concentrate of fractions 8-12; 436.89 U) of IEC, and fraction II (10-20% polyethylene glycol; 307.31 U) of PGF. Total exopeptidase activity of these fractions was highest in fraction II (2,704.70 U) of GFC, fraction III (2,110.50 U) of ASF, fraction III (1,605.60 U) of PGF, and fraction II (concentrate of fractions 38-44; 1,196.22 U) of IEC. These results showed that fraction II of GFC had the highest activity toward both exopeptidase and endoprotease, with exopeptidase activity being 3.21 times higher than of endoprotease. These results suggest cuttlefish HP could be used as a potential source for the extraction of exopeptidase, an enzyme capable of catalyzing the cleavage of N- and C-terminal amino acids in polypeptides, Like endoprotease, the most efficient method for separating exopeptide-active fractions was GFC.
A large quantity of cuttlefish has been caught in the adjoining Korea seas. In South Korea, the cuttlefish is a popular seafood, with an output of 4,109 metric tons in 2010 (Agriculture and Fishery Statistics Department, 2010). The cuttlefish processing industry generates large amounts of solid by-products, including viscera, skin, and bone, which can cause major environment issues (Souissi et al., 2008). Among these solid by-products, viscera represents 14-16% of the original material and constitutes an important source of protein, lipids, and enzymes. Thus, using this by-product to act as a source for the extraction of important enzymes, such as endoprotease and exopeptidase, would be advantageous.
Proteases constitute the most important group of industrial enzymes used in the world today and account for ~50% of the total industrial enzyme market (Rao et al., 1998; Bougatef et al., 2007). These enzymes have diverse applications in a wide variety of industries, including the detergent, food, agrochemical, and pharmaceutical industries (Gupta et al., 2002; Bougatef et al., 2007). In the food industry, proteases are most extensively used for improving the quality, stability, and solubility of foods produced by the baking, brewing, and cheese-making processes, as well as meat processing (Haard, 1990; Heu et al., 2003). However, the enzymatic hydrolysates of various proteins frequently exhibit a bitter taste caused by bitter peptides, which limits their utilization in the food industry (Matoba et al., 1970; Clegg et al., 1974; Bumberger and Belitz, 1993; Izawa et al., 1997). Since bitterness is closely correlated with the hydrophobicity of the peptides (Clegg et al., 1974; Izawa et al., 1997), several attempts have been made to reduce the bitterness by hydrolyzing bitter peptides with exopeptidases (Minagawa et al., 1989; Umetsu et al., 2003). Although many industrial proteases have been developed, a limited number of these commercial peptidases exist, including aminopeptidase and carboxypeptidase (Deejing et al., 2005).
The efficient isolation of endoprotease and exopeptidase from cuttlefish hepatopancreas (HP), requires the application of extraction, fractionation, and purification procedures. Among these procedures, fractionation is expected to significantly affect enzyme activity. In recent years, several studies have reported methods for the efficient extraction of hydrolysates (Kechaou et al., 2009), collagen and gelatin (Nagai et al., 2001; Aewsiri et al., 2009; Hoque et al., 2010), functional lipids (Park et al., 2011), media (Souissi et al., 2008), and calcium (Ivankovic et al., 2009) from cuttlefish-processing by-products, such as skin, viscera, and bone. However, an efficient method for the extraction of exopeptides, such as aminopeptidase and carboxypeptidase, has yet to be reported.
Here, we report the enzymatic characterization endoprotease- and exopeptidase-active cuttlefish HP fractions isolated by various separation methods, including ammonium sulfate fractionation (ASF), polyethylene glycol fractionation (PGF), ion exchange chromatography (IEC), and gel filtration chromatography (GFC).
Frozen cuttlefish
Endoprotease- and exopeptidase-active fractions were isolated using ammonium sulfate and polyethylene glycol purchased from Katayama Chemical Co. (Osaka, Japan) and Yacuri Pure Chemical Co. Ltd. (Kyoto, Japan), respectively. DEAE 650M and Sephacryl S300 were purchased from Tosoh Co. Ltd. (Tokyo, Japan) and Pharmacia (Uppsala, Sweden), respectively. L-leucine-
>
Preparation of the crude extracts (CE)
Neocoleoidea CE was prepared by partially thawing frozen cuttlefish before homogenizing the HP with 3 volumes (v/w) of deionized water. Enzyme activation was performed by incubating the homogenates for 4 h at 20℃, while stirring every 30 min, and centrifuging the solutions at 12,000
The protein concentration of the CE was measured as previously described (Lowry et al., 1951) using bovine serum albumin as a standard protein.
>
Activity of endoprotease and exopeptidase
Endoprotease activity was assessed using an azocasein as the substrate by a method described by Starky (1977) with some modifications. The CE (100 μL) was mixed with 300 μL of 1% azocasein in 1.6 mL of 0.1 M sodium phosphate (pH 6.0) prior to incubation at 40℃ for 1 h. The reaction was stopped by the addition of 2 mL of 5% trichloroacetic acid solution, and the mixture was set before centrifuging at 146
LeuPNA was used to assess exopeptidase activity using the method reported by Garcia-Carreno and Haard (1993) with slight modification. Briefly, CE (100 μL) were mixed with 100 μL of LeuPNA in 2 mL of 0.1 M sodium phosphate buffer (pH 6.0) and incubated at 40℃ for 1 h. The reaction was stopped by the addition of 0.3 mL of 33% acetic acid solution and allowed to set before centrifuging at 146
>
Fractionation of endoprotease and exopeptidase
The CE were fractionated by ASF, PGF, IEC or GFC to obtain endoprotease- and exopeptidase-active fractions from cuttlefish HP.
The CE were fractionated by adding ammonium sulfate to the solutions in the range of 0-20%, 20-40%, 40-60%, and 60- 80% saturation. These fractions were collected by centrifugation (12,000
The CE was fractionated by adding polyethylene glycol to the extract in the range of 0-5%, 5-10%, 10-20% and 20- 40%. Fractions were collected by centrifugation (12,000
A Toyopearl DEAE 650M column (1.6 × 20 cm) was equilibrated with 10 mM sodium phosphate buffer (pH 7.0) and washed with the same buffer until the conductivity of the eluent equaled that of the buffer. For isolation of the exopeptidase- active fraction, the CE were applied to the column and eluted before the addition of 110 mL of solution containing an increasing NaCl gradient (0 M, 0.5 M and 1.0 M) at a flow rate of 30 mL/h. Protein content and protease activity were measured by monitoring absorbance at 280 nm and 410 nm, respectively. Exopeptidase-active fractions (4 mL/tube) were collected and centrifuged at 3,000 rpm for 30 min using an Amicon Bioseparations Centriplus centrifugal filter devices (Millipore, Billerica, MA, USA) to concentration and dialyze the fraction. The supernatants were stored at -25℃ until enzymatic characterization.
A Sephadex S-300 column (11.6 × 20 cm) was equilibrated with 10 mM sodium phosphate buffer (pH 7.0) and washed with the same buffer until the conductivity of the eluent and the buffer was equal. Isolation of the exopeptidase-active fraction
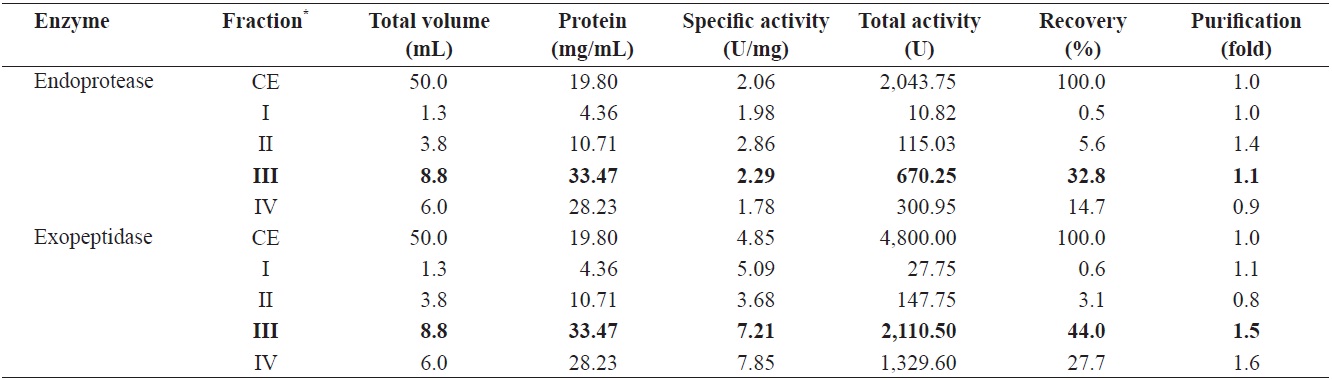
Endoprotease and exopeptidase activities of fractions obtained from the CE of cuttlefish Sepia officinalis hepatopancreas by the ammonium sulfate fractionation toward azocasein and LeuPNA as substrates
was accomplished by applying the column and eluting prior to separation at a flow rate of 12 mL/h. Protein content and protease activity were measured by reading the absorbances at 280 nm and 410 nm, respectively. Exopeptidase-active fractions (4 mL/tube) were collected and centrifuged at 3,000 rpm for 30 min using an Amicon Bioseparations Centriplus centrifugal filter devices (Millipore), which concentrated and dialyzed the solution. The supernatants were stored at -25℃ until use.
This study focused on the efficient isolation of endoprotease- and exopeptidase-active fractions from cuttlefish HP using various fractionation methods, including ASF, PGF, IEC and GFC.
>
Protease activity ASF fractions
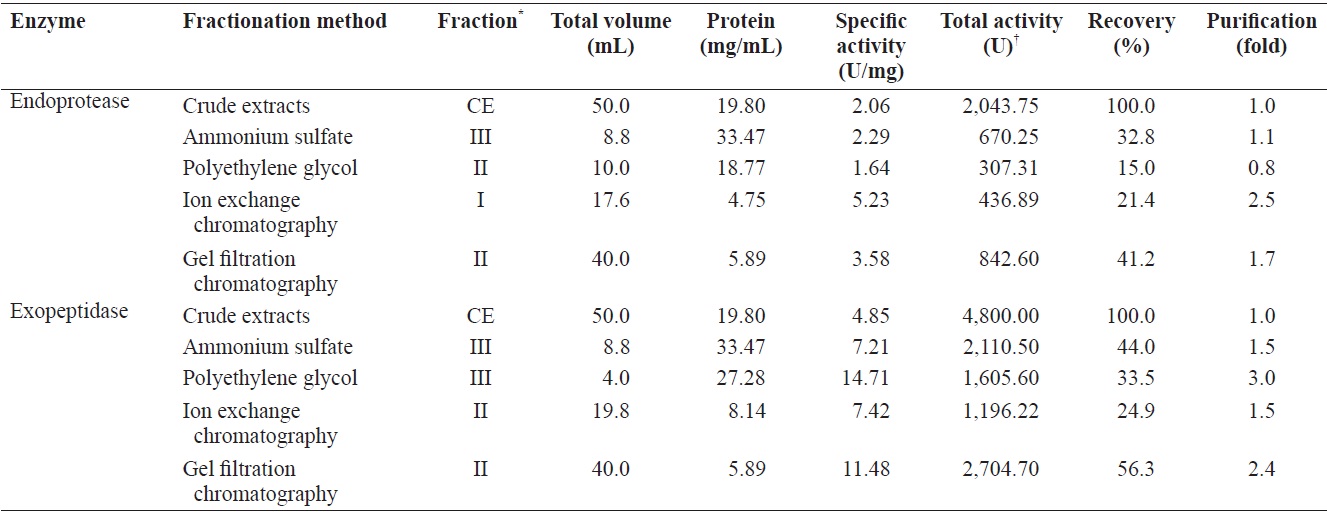
To identify the optimal ASF condition for isolating endoprotease- and exopeptidase-active fractions from cuttlefish HP, the CEs were fractionated with increasing concentration of ammonium sulfate. The fractions were designated as fraction I (0-20% ammonium sulfate), II (20-40% ammonium sulfate), III (40-60% ammonium sulfate) and IV (60-80% ammonium sulfate).
Table 1 shows endoprotease and exopeptidase activities, as well as the fractional yield obtained from the CE of cuttlefish HP using various concentrations of ammonium sulfate. Specific activity and endoprotease-specific activities were highest in fraction II (2.86 U/mg and 1.4-fold, respectively), followed by fraction III (2.29 U/mg and 1.1-fold), fraction I (1.98 U/mg and 1.0-fold), and fraction IV (1.78 U/mg and 0.9-fold). These results indicate that the purification of endoprotease from the CE was largely affected by the concentration of an ammonium sulfate, with the highest amount of activity observed in fractions containing lower concentrations of ammonium sulfate (
The total recovery of endoprotease activity was of 53.6% following fractionation of the CE by ASF. Recovery of endoprotease activity in fraction III was 32.8%, which constituted ~61% of the total recovery. Endoprotease activity in CE fractions was the highest in the fraction III (670.25 U), followed by the fraction IV (300.95 U), II (115.03 U), and I (10.82 U). These results indicate that the majority of endoprotease activity was contained in fraction III.
Bougatef et al. (2007) reported that endoprotease fractions, isolation from the CE of sardine
Specific activity and purification of exopeptidase in fractions from cuttlefish HP CE were the highest in fraction IV (7.85 U/mg and 1.6-fold, respectively), followed by fraction III (7.21 U/mg and 1.5-fold), I (5.09 U/mg and 1.1-fold), and II (3.68 U/mg and 0.8-fold). According to these results, the purification of exoprotease was affected by the concentration of ammonium sulfate used during ASF fraction of cuttlefish HP CE.
Total recovery of exopeptidase activity from cuttlefish HP CE fractions was 75.4%. The recovery of fraction III was 44.0% and that of fraction IV was 27.7%, which together constituted
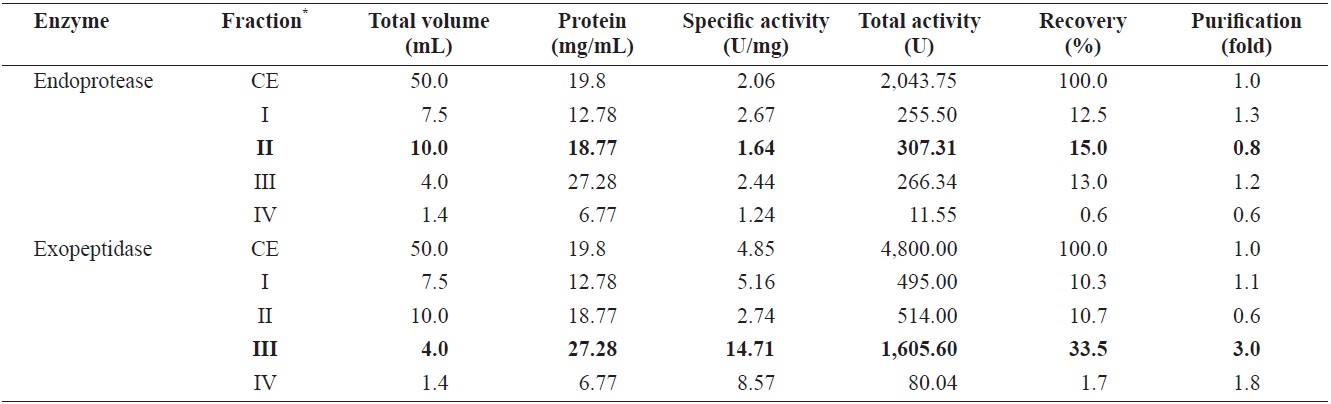
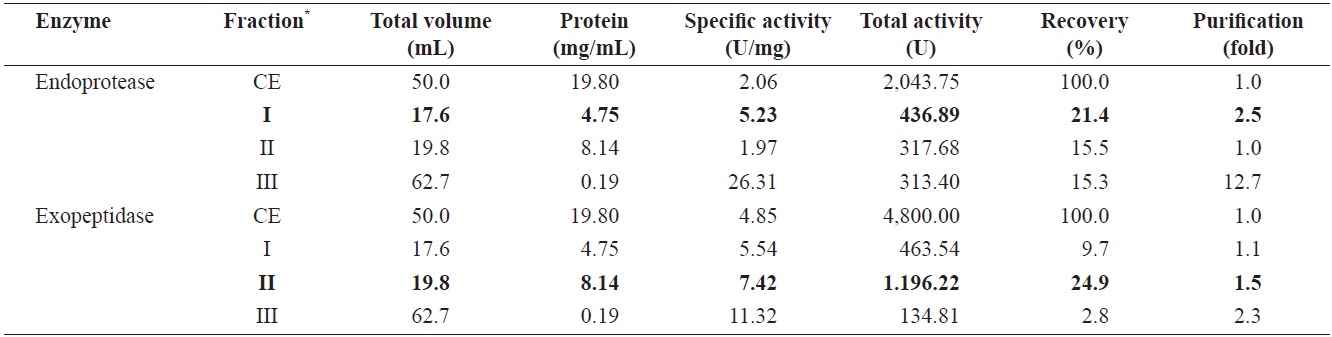
Endoprotease and exopeptidase activities of fractions obtained from the CE of cuttlefish Sepia officinalis hepatopancreas by the polyethylene glycol fractionation toward azocasein and LeuPNA as substrates
~95% of the total recovery. Total exopeptidase activity was highest in fraction III (2,110.50 U), followed by fraction IV (1,329.60 U), II (147.75 U), and I (27.75 U). These results indicate that fraction III contained the highest exoprotease activity and recovery. Indeed, Raksakulthai and Haard (1999) reported that purification and recovery of exopeptidase of from ASF fractionation of squid
Fraction III showed both the highest total endoprotease activity value (670.25 U) using an azocasein as a substrate and the highest exopeptidase value (2,110.50 U) using LeuPNA as a substrate. The toal activity of exopeptidase in fraction III was 3.15 times higher than that of endoprotease. These results suggest that cuttlefish HP could be used as a potential source for the extraction of exopeptidase, which is an enzyme capable of catalyzing the cleavage of N- or C-terminal amino acids in polypeptides, ASF appears to be an efficient method for the isolation of exopeptidase-active fraction from the CE of cuttlefish HP.
>
Protease activity of PGF fraction
Optimizing the conditions for fractionation of endoprotease and exopeptidase-activities from cuttlefish HP CE was performed using increasing concentrations of polyethylene glycol, Fractions were designated as fraction I (0-5% polyethylene glycol), II (5-10% polyethylene glycol), III (10-20% polyethylene glycol), and IV (20-40% polyethylene glycol).
Table 2 shows endoprotease and exopeptidase activities, as well as the fractional yield obtained from the CE of cuttlefish HP using various concentrations of polyethylene glycol. Specific activity and endoprotease purification from the CE fracrespections were highest in fraction I (2.67 U/mg and 1.3-fold, respectively), followed by fraction III (2.44 U/mg and 1.2-fold), II (1.64 U/mg and 0.8-fold), and IV (1.24 U/mg and 0.6-fold). According to these results, the purification of endoprotease from CE was largely affected by the concentration of polyethylene glycol, but the activity was independent of polyethylene glycol concentration.
The total recovery of endoprotease activity from the CE fractionation was 41.1%, Individual fractions II, III, and I had endoprotease activity of 15.0%, 13.0%, and 12.5%, respectively, which corresponded respectively to ~36.5%, 31.6%, and 30.4% of the total activity recovered. Endoprotease activity was the highest in fraction II (370.31 U), followed by fraction III (266.34 U), I (255.50 U), and IV (11.55 U). These results indicate that endoprotease activity and recovery was highest in the fraction containing 5-10% polyetylene glycol, followed by the fraction containing 10-20% polyetylene glycol and that containing 0-5% polyetylene glycol. Thus, endoprotease- active fraction could be isolated using 0-20% polyethylene glycol.
Specific activity and purification of exopeptidase from the CE fractions were the highest in the fraction III (14.71 U/mg and 3.0-fold, respectively), followed by fraction IV (8.57 U/ mg and 1.8-fold), I (5.16 U/mg and 1.1-fold), and II (2.74 U/ mg and 0.6-fold). These results suggest that the purification of exopeptidase from the CE was influenced by concentration of polyethylene glycol, with higher concentration (>10%) yielding higher activities and purities.
Total recovery of exopeptidase from CE fractions of cuttlefish HP was 56.2%. The recovery of exopeptidase activity in fraction III was 33.5%, which constituted ~60% of the total recovery. Total exopeptidase activity was the highest in fraction III (1,605.60 U), followed by fraction II (514.00 U), I (495.00 U), and IV (80.04 U). These results indicate exoprotease activity is highest in fraction III, suggesting exoprotease activity could be effectively fractionated using 10-20% polyethylene glycol. The highest total endoprotease activity (307.31 U) was observed in fraction II, with the highest exopeptidase activity
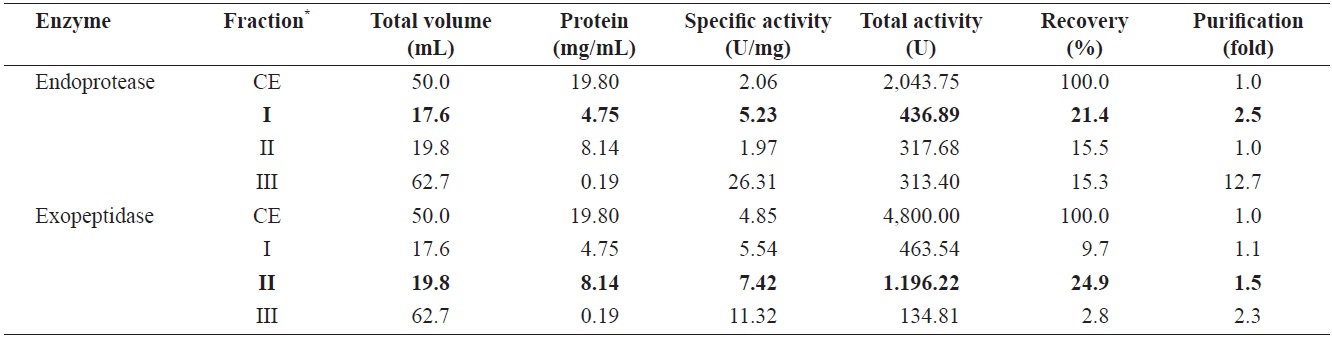
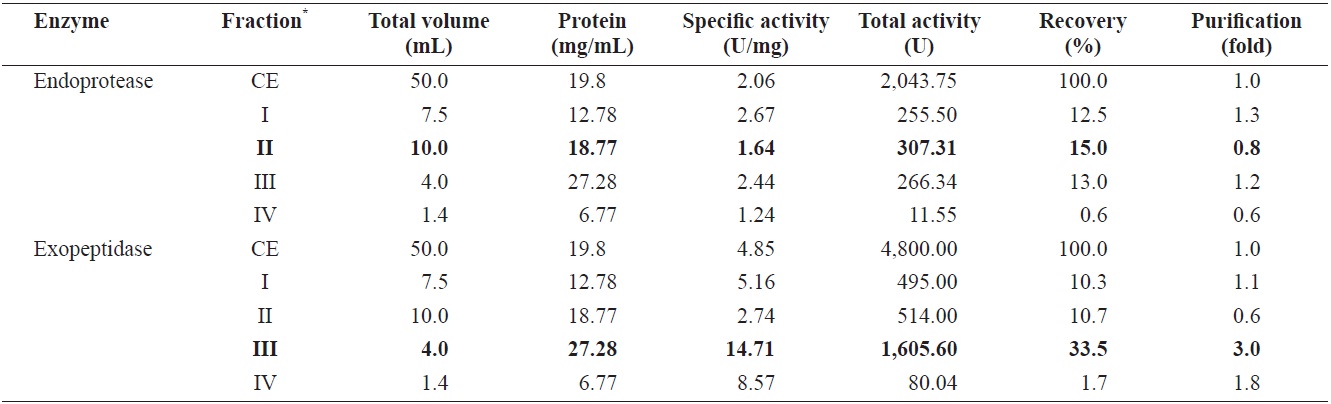
Endoprotease and exopeptidase activities of fractions obtained from the CE of cuttlefish Sepia officinalis hepatopancreas by the DEAE-650M ion exchange chromatography toward azocasein and LeuPNA as substrates
(1,605.60 U) present in fraction III. The total activity of exopeptidase in fraction III was 5.22 times higher than that of endoprotease in fraction II. These results suggest that cuttlefish HP could be used as a potential source for the extraction of exopeptidase and that PGF is an efficient method for isolating exopeptidase-active fractions from the CE of cuttlefish HP.
>
Protease activity of IEC fractions
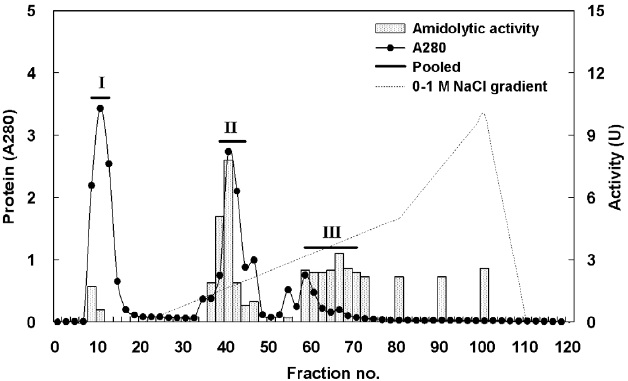
Fig. 1 shows a chromatogram of the optimized IEG conditions used to isolate endoprotease- and exopeptidase-active fractions from cuttlefish HP using a DEAE-650M ion exchange resin. Based on the protein concentration and enzymatic activities of endoprotease and exopeptidase, fractions were pooled, concentrated, and designated as fraction I (concentrates of fraction 8-12), II (concentrates of fraction 38-44), and III (concentrates of fraction 58-70).
Endoprotease and exopeptidase activities, as well as the fractional CE yield of cuttlefish HP separated by IEC, are shown in Table 3. Specific activity and endoprotease purity was highest in fraction III (26.31 U/mg and 12.7-fold, respectively), followed by fraction I (5.23 U/mg and 2.5-fold) and II (1.97 U/mg and 1.0-fold). According to the results, the purification of endoprotease from the CE was largely affected by the ionic strength of the resin. These results indicate that IEC is an efficient method for obtaining endoprotease-active fraction from the CE of cuttlefish HP.
Total recovery of endoprotease from CE fractions of the cuttlefish HP was 52.2%, Among the three pooled fractions, endoprotease recoveries for fraction I and II were 21.4% and 15.5%, respectively, which constituted approximately 41.0% and 29.7%, respectively, of the total recovery. Total activity of endoprotease in the CE fractions was the highest in fraction I (436.89 U), followed by fraction II (317.68 U) and III (313.40 U). These results show the highest endoprotease activity in fraction I, which indicates that endoprotease-active fractions can be isolated by IEC using a DEAE-650M ion exchange resin.
Fu et al. (2005) reported that an alkaline protease-active fraction from the CE of sea cucumber
Specific activity and purification of exopeptidase from CE fractions, of cuttlefish HP were the highest in fraction III (11.32 U/mg and 2.3-fold, respectively), followed by fraction II (7.42 U/mg and 1.5-fold), and I (5.54 U/mg and 1.1-fold). These findings suggested that IEC may be an efficient method for obtaining exoprotease-active fractions from the CE of cuttlefish HP.
Total recovery of exopeptidase in CE fractions was 37.4%, with the individual recovery of fraction II being 24.9%, which constituted ~66.6% of the total recovery. Exopeptidase activity was highest in fraction II (1,196.22 U), followed by fraction I (463.54 U) and III (134.81 U). These results show exopeptidase to be active and present in high amounts in fraction II. Thus, the exopeptidase-active fraction could be effectively isolated from the CE using IEC.
Vo et al. (1983) reported that a protease-active fraction from the CE of sardine viscera could be isolated by IEC, with a recovery and purity of 79% and 36-fold, respectively. Umetsu et al. (2003) also reported that a protease-active fraction from the CE of scallop
Total activity was highest in fraction I (436.89 U) for endoprotease and in fraction II (1,196.22 U) for exopeptidase. The total activity of exopeptidase was 2.74 times higher than that of endoprotease. These results suggest that cuttlefish HP may be a potential source the extraction of exopeptidase, and DEAE-650M is an efficient resin for isolating exopeptidase-active fractions from the CE of cuttlefish HP using IEC.
>
Protease activity of fractions obtained by GFC
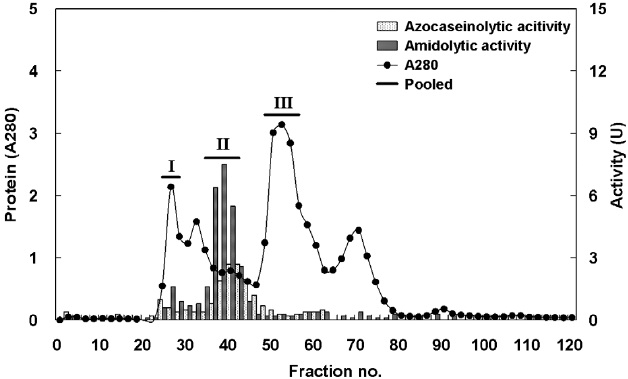
Fig. 2 shows the optimized isolation of endoprotease- and exopeptidase-active fractions from the CE of cuttlefish HP by GFC using a Sephacryl S-300 resin. Based on the chromatogram, endoprotease and exopeptidase, activity levels and protein concentration values, fractions were designated as fraction I (concentrate of fractions 24-28), II (concentrate of fractions 38-42), and III (concentrate of fractions 48-56).
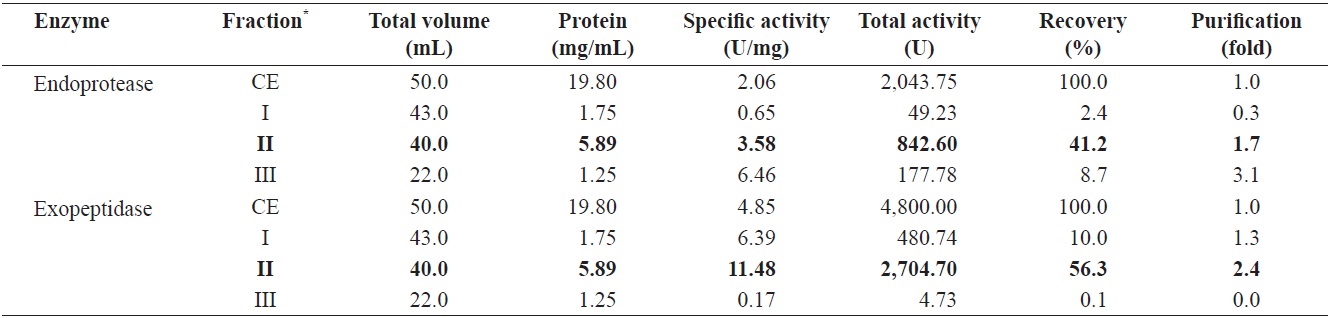
Endoprotease and exopeptidase activities, as well as the fractional yield from the CE of cuttlefish HP isolated by GFC are shown in Table 4. Specific activity and endoprotease purity are highest in fraction III (6.46 U/mg and 3.1-fold, respectively), followed by fraction II (3.58 U/mg and 1.7-fold) and I (0.65 U/mg and 0.3-fold). According to these results, the purification of endoprotease from the CE was significantly affected by difference in molecular weight. These results suggest that GFC is an efficient method for the separation of endoprotease-active fractions from the CE of cuttlefish HP.
Total recovery of endoprotease from GFC fractions was 52.3%. Among the three fractions, recovery of endoprotease in fraction II was 41.2%, which constituted ~78.8% of the total recovery. Total activity of endoprotease in the GFC fractions was highest in fraction II (842.60 U), followed by fraction III (177.78 U) and I (49.23 U). The total endoprotease activity and recovery results showed the highest value in fraction II. Thus, endoprotease-active fractions could be effectively isolated using Sephacryl S-300 resin.
Kishimura et al. (2005) reported that a protease-active fraction from the CE of anchovy
Total recovery of exopeptidase by GFC fractionation was 66.4%. Recovery of exopeptidase in fraction II was 56.3%, which constituted ~81.2% of the total recovery. Total exopeptidase activity was highest in the fraction II (2,704.70 U), followed by fraction I (480.74 U). These results indicate the total activity and recovery of exopeptidase of fractions was highest in fraction II. Thus, the exopeptidase-active fraction could be effectively isolated by GFC using a Sephacryl S-300 resin.
Total activity was highest for both endoprotease (842.60 U) and exoprotease (2,704.70 U) in fraction II, with the total activity of exopeptidase being 3.21 times higher than that of endoprotease in this fraction. These results suggest that cuttlefish HP could be used as a potential source for the extraction of exopeptidase and that GFC fractionation using a Sephacryl S-300 resin is an efficient method for the isolation of exopeptidase- active fractions from the CE of cuttlefish HP.
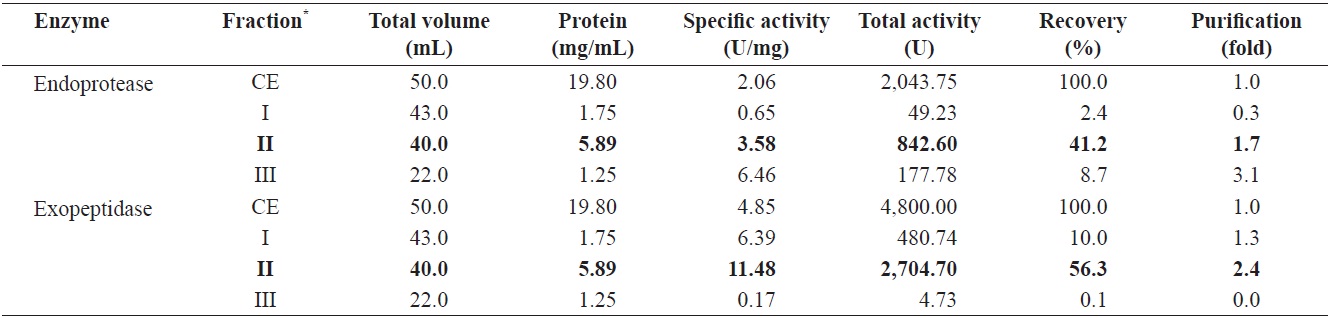
Endoprotease and exopeptidase activities of fractions obtained from the CE of cuttlefish Sepia officinalis hepatopancreas by the Sephacryl S-300 gel filtration chromatography toward azocasein and LeuPNA as substrates
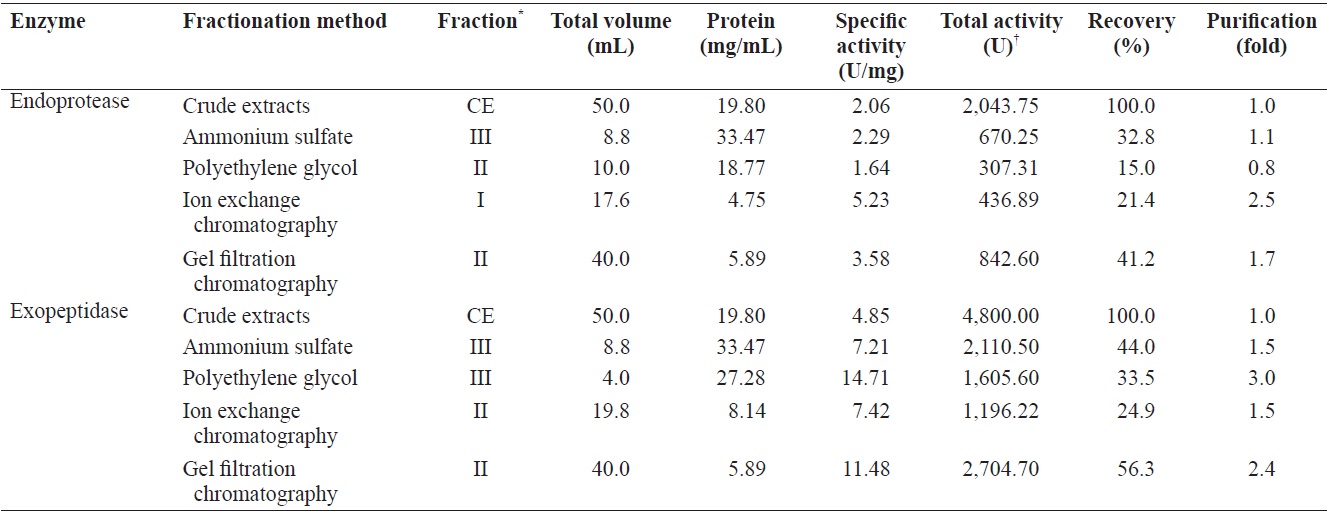
Comparison of enzyme activities of fractions from the CE of cuttlefish Sepia officinalis hepatopancreas obtained by different fractionation methods toward azocasein and LeuPNA as substrates
Fraction III of ASF contained the highest total activity for both azocasein and LeuPNA, while fraction II and III collected by PGF had the highest activity for the respective substrates. Fraction I and II of IEC had the highest azocasein and LeuPNA total activity, respectively, while the highest activities for GFC were both found in fraction II. Endoprotease and exopeptidase activities, as well as fractional yield, are shown in Table 5 for each of the fractionation methods. Specific activity and endoprotease purification were the highest in fraction I (5.23 U/mg and 2.5-fold, respectively) of IEC, followed by fraction II (3.58 U/mg and 1.7-fold) of GFC, fraction III (2.29 U/mg and 1.1-fold) of ASF, and fraction II (1.64 U/mg and 0.8-fold) of PGF. Total endoprotease activity was the highest in the fraction II (842.60 U) of GFC, followed by fraction III (670.25 U) of ASF, fraction I (436.89 U) of IEC, and fraction II (307.31 U) of PGF. These results suggest that GFC is the most efficient method for separating endoprotease-active fraction from the CE of cuttlefish HP.
Specific activity and purification of exopeptidase were the highest in fraction III (14.71 U/mg and 3.0-fold, respectively) of PGF, followed by fraction II (11.48 U/mg and 2.3-fold) of GFC, fraction II (7.42 U/mg and 1.5-fold) of IEC, and fraction III (7.21 U/mg and 1.5-fold) of ASF. Total activity toward exopeptidase was highest in fraction II (2,704.70 U) of GFC, followed by fraction III (2,110.50 U) of ASF, fraction III (1,605.60 U) of PGF, and fraction II (1,196.22 U) of IEC.
Total activity results suggest that GFC is the most efficient method for separating exopeptidase-active fractions from the CE of cuttlefish HP. Fraction II of GFC, which had the highest total activity toward both exopeptidase and endoprotease, had 3.21 times higher total activity toward exopeptidase than endoprotease. These results suggest that cuttlefish HP could be used as a potential source for extracting exopeptidase, with GFC being the most efficient method studied here for separating exopeptidase-active fractions from the CE of cuttlefish HP.