Yellow croaker Larimichthys polyactis muscle oil was extracted using an environmental friendly solvent, supercritical carbon dioxide (SC-CO2), in a semi-batch flow extraction process. SC-CO2 was applied at temperature 35℃ to 45℃ and 150℃ to 250℃ bar of pressure. The flow rate of CO2 (27.79 g/min) was constant throughout the entire 1.5 h extraction period. The oil extraction yield was influenced by the physical properties of SC-CO2 at different temperatures and pressures. The extracted oil was analyzed by gas chromatography to determine the fatty acid composition. According to our results, the SC-CO2 extracted oil was high in eicosapentaenoic acid and docosahexaenoic acid. In addition, the SC-CO2 extracted oil showed greater stability than n-hexane extracted oil based on the peroxide value and acid value. Thus, the quality of yellow croaker oil obtained by SC-CO2 extraction was slightly higher than that of oil obtained by n-hexane extraction.
Yellow croaker
Generally, lipids are extracted using organic solvents, and several methods have been developed for extracting fish oils with varying yields. The extraction and purification of lipids by conventional methods, including hexane extraction, vacuum distillation, urea complexation, and conventional crystallization, have the disadvantage of requiring processing at high temperatures, resulting in the decomposition or degradation of these heat-labile compounds. Moreover, organic solvents are harmful to human health and the environment (Staby and Mollerup 1993; Hultin, 1994; Sahena et al., 2010).
Supercritical fluid extraction (SFE) is an efficient alternative for the extraction of natural substances from foods (Mendes et al., 2003; Sun and Temelli, 2006). In recent years, the use of SFE for the removal of organic compounds from different liquid and solid matrices has attracted a great deal of attention. This technique has some advantages over conventional separation techniques, largely due to the unique physical properties of supercritical fluids. Supercritical fluid separation using carbon dioxide (CO2) as the solvent carries offers
potential advantages because it is nonflammable, nontoxic, inert to most materials, inexpensive, and can be used under mild operational conditions (Ge et al., 2002). Moreover, it has been widely used in many industrial applications, including the extraction of fish oil for PUFAs, the decaffeination of coffee, the extraction of hops and carotenoids, the synthesis of polymers, and the formation and purification of nanoparticles (Temelli et al., 1995; Lim et al., 2002; Kopcak and Mohamed, 2005; Letisse et al., 2006; Machmudah et al., 2006; Rubio- Rodriguez et al., 2008; Sahena et al., 2010). Several studies have investigated the extraction of oils rich in PUFAs using supercritical carbon dioxide (SC-CO2). This process, which has a negligible environmental impact, represents a potential tool for changing the relative concentration of various lipid moieties (Eisenbach, 1984; Rizvi et al., 1998; Temelli et al., 1995; Perretti et al., 2003).
The objective of this study was to extract oil from yellow croaker muscle using SC-CO2 and hexane. The fatty acid compositions of the oils produced using these different extraction processes were then analyzed, and the stability of the SC-CO2 and hexane extracted oils was compared.
Yellow croaker (average weight, 17.5 cm; sample length, 158 g) was collected from Busan Cooperative, Fish Market (Busan, Korea). Muscle was separated and washed thoroughly with cold water. Pure CO2 (99.99%) was supplied by KOSEM (Yangsan, Korea). All reagents used in this study were of analytical or high-performance liquid chromatography grade.
The yellow croaker muscle samples were dried in a freeze-dryer for about 72 h, then crushed with a mechanical blender and sieved through a mesh size, 2 mm. The samples were stored at -20℃ prior to SC-CO2 or hexane extraction.
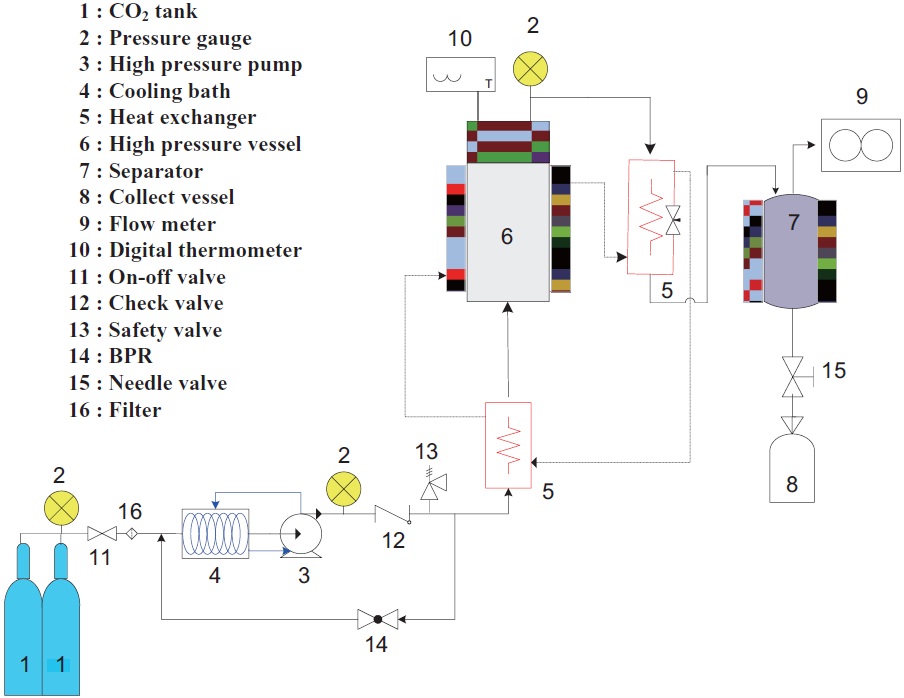
A laboratory-scale SFE process was performed (Fig. 1). The apparatus can be operated at pressures up to 300 bar. A total of 30 g of freeze dried raw yellow croaker muscle was placed in a 200 mL stainless steel extraction vessel with a thin layer of cotton at the bottom. A second layer of cotton was applied across the top of the sample. CO2 was pumped at a constant pressure into the extraction vessel with a high pressure pump set at the desired pressure. A back pressure regulator was used to control the pressure of CO2. The extraction temperature was maintained using a water bath connected to the extraction vessel. The flow rate and accumulated gas volume passing through the apparatus were measured by a gas flow meter. The effects of temperature and pressure on lipid extraction from yellow croaker were measured at 35-45℃ and 150-250 bar. The flow rate of CO2 (27.79 g/min) was held constant throughout the 1.5 h extraction period. A modifier was not used. The extracted oil was collected in a vial and stored at -20℃ until further analysis.
The extraction procedure was carried out with or without Soxhlet apparatus with hexane as the solvent. During use of the Soxhlet apparatus, 2 g of freeze dried raw yellow croaker muscle was placed in an extraction thimble and the extraction process was run for 24 h, until the color of the condensed solvent at the top of the apparatus was clear. For extraction without the Soxhlet apparatus using, 40 g of freeze dried raw yellow croaker muscle was placed with 200 mL of hexane into a beaker and stirred for 24 h by a magnetic stirrer at 45℃ and 250 rpm. After extraction, the hexane solution was evaporated in a rotary vacuum evaporator at 40℃. The extracted oil was collected in a vial and stored at -20℃ until further analysis.
>
Determination of the fatty acid composition
Gas chromatographic analysis was carried out to determine the fatty acid composition of yellow croaker muscle oil obtained using SC-CO2 or hexane (without Soxhlet apparatus). Gas chromatography-mass spectrometry was performed by a 6890 Agilent Technologies (Wilmington, DE, USA) gas chromatograph with a fused silica capillary column (length, 100 m; internal diameter, 25 mm; length of film, 0.2 μm; Supelco, Bellefonte, PA, USA). Fatty acid methyl esters were prepared according to the Official Method and Recommended Practices of the AOCS Ce 2-66 (1998). Nitrogen was used as a carrier gas (1.0 mL/min). The oven was programmed to start at a constant temperature of 130℃ for 3 min, and then to increase 240℃ at a rate of 4℃/min and hold at 240℃ for 10 min. The injector and detector were both set at 250℃. Fatty acid methyl esters were identified by comparison of the retention time with a standard fatty acid methyl ester mixture (Supelco).
>
Measurement of oil stability
Several parameters may be used to determine the deterioration of oil. In this study, oil deterioration was monitored by evaluating the acid value (AV) and peroxide value (POV).
The AV was assessed according to the Official Method and Recommended Practices of the AOCS Cd 3d-63 (1998). A total of 1 g of sample was dissolved in 100 mL of ether:ethanol (1:1) and shaken. Next, four drops of phenolphthalein were added as an indicator. The solution was titrated with 0.1 N KOH-ethanol until it turned pink. The AV, expressed as mg KOH per g of sample, was calculated using the formula.
where A is the volume of KOH-ethanol solution of the titration (mL), F is concentration of KOH-ethanol, S is the mass of oil (g), and 56.11 is the molecular weight of KOH.
The POV was determined according to the Official Method and Recommended Practices of the AOCS Cd 8-53 (1998). A total of 1.0 g of yellow croaker muscle oil was dissolved in 6 mL of acetic acid-chloroform (3:2). Next, 0.1 mL of saturated KI was added, and the mixture was allowed to stand with occasional shaking for 1 min. Distilled water (6 mL) was immediately added to the solution. The solution was titrated with 0.1 N sodium thiosulfate until the yellow color of the iodine had almost disappeared. Next, 0.4 mL of starch indicator solution was added and again titrated until the blue color disappeared. A blank determination was conducted with the same procedure. The POV, expressed as milliequivalents (meq) peroxide/ 1,000 g sample, was calculated using the equation
where S is the volume of the titrant (mL), B is the volume of titrant blank (mL), N is the normality of the sodium thiosulfate solution, and M is the mass of the sample (g).
All experiments were carried out in duplicate. The data are expressed as the mean ± standard deviation. Differences (
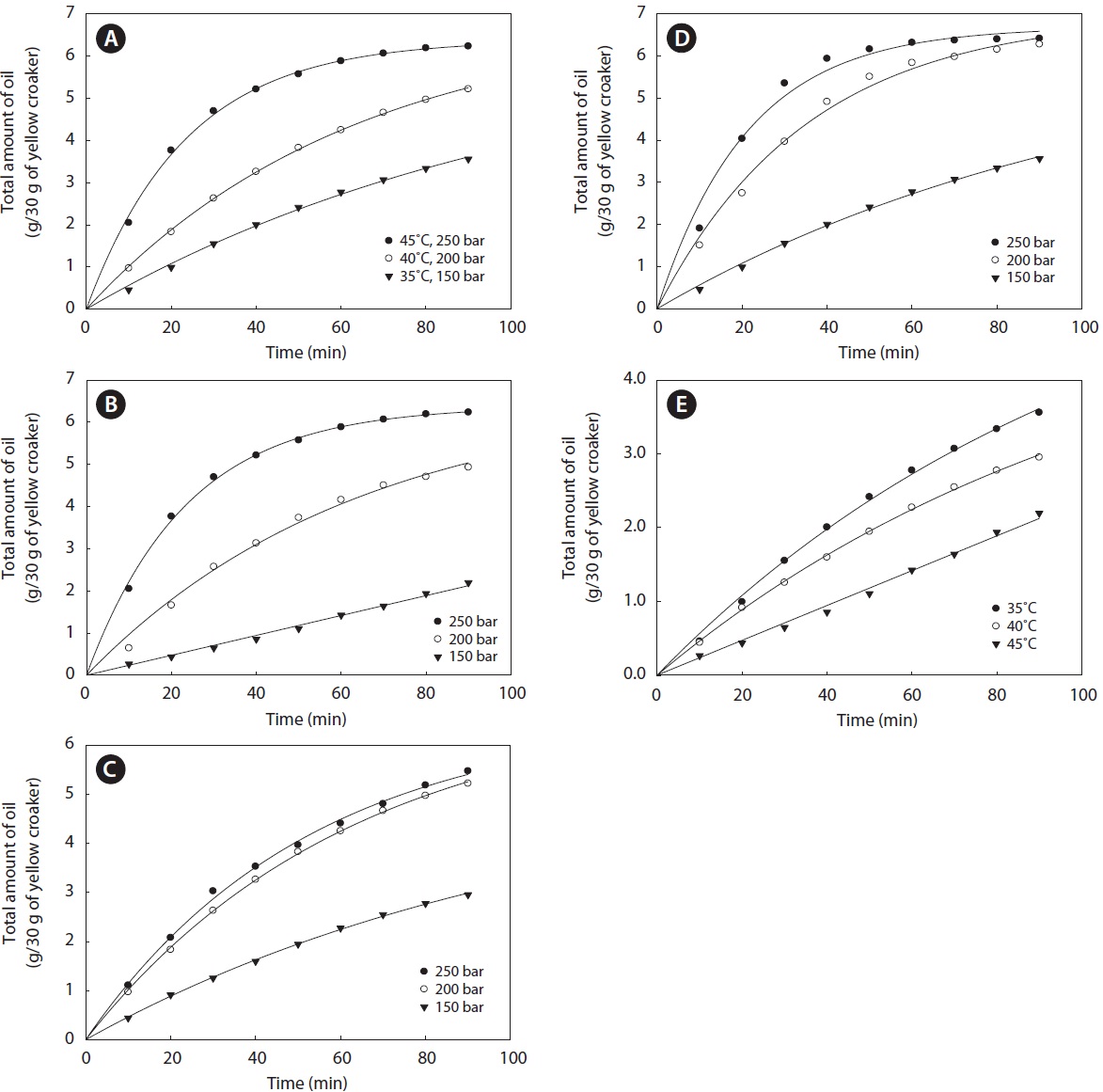
The extraction curves for yellow croaker muscle oil prepared using SC-CO2 at different temperatures (35℃, 40℃, and 45℃) and pressures (150, 200, and 250 bar) are shown in Fig. 2. The highest oil yield obtained by SC-CO2 extraction was 6.41 ± 0.08 g/30 g of yellow croaker muscle at 35℃ and 250 bar. The temperature and pressure applied greatly affected the oil solvating power of SC-CO2, and hence, the yield. Depending on the pressure and temperature, the amount of oil extracted increased as the mass of CO2 increased. The amount of extracted oil per solvent (CO2) mass used increased constantly throughout the extraction period, until almost all of the oil was extracted. The slope of the extraction curve (35℃
and 250 bar) indicated that SC-CO2 extracted almost all of the yellow croaker muscle oil. At a constant temperature, the amount of oil extracted from yellow croaker muscle increased with the pressure. Due to the increase in pressure, the density, and hence, the solvating power of SC-CO2 increased. At a constant pressure, the amount of oil extracted from yellow croaker muscle decreased as the temperature increased. The solvating power of supercritical fluids decreases with a rise in temperature because the density decreases dramatically as the temperature increases at low pressures. The effect of pressure can be attributed to the increase in solvating power and the strengthening of intermolecular interactions (Morita and Kajimoto, 1990; Bai et al., 1997; Bulgarevich et al., 2002). Similar results were found in the extraction of oil from green coffee (De Azevedo et al., 2008) and boiled anchovy (Park et al., 2008).
>
Comparison of the oil yield by SC-CO2 and hexane extraction
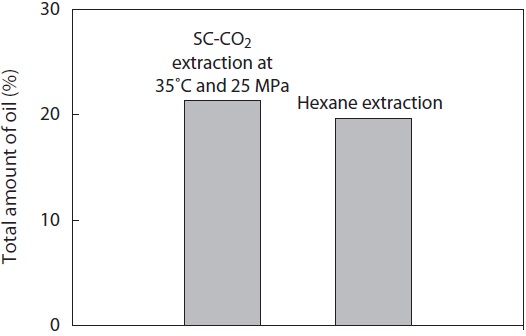
As shown in Fig. 3, the total amount of oil obtained from yellow croaker muscle by Soxhlet extraction using hexane was 19.70 ± 0.42% (w/w freeze dried raw sample), while the highest total amount of oil obtained by SC-CO2 extraction was 21.36 ± 0.28% at 250 bar and 35℃ (Fig. 2). Considering that the extraction of oil using SC-CO2 at 250 bar and 35℃ was complete, the yield by hexane extraction was almost 92.23%. The observed difference in maximum yield may have been
due to variations in the processing unit, operating conditions, sample size, or percentage of lipid in the sample.
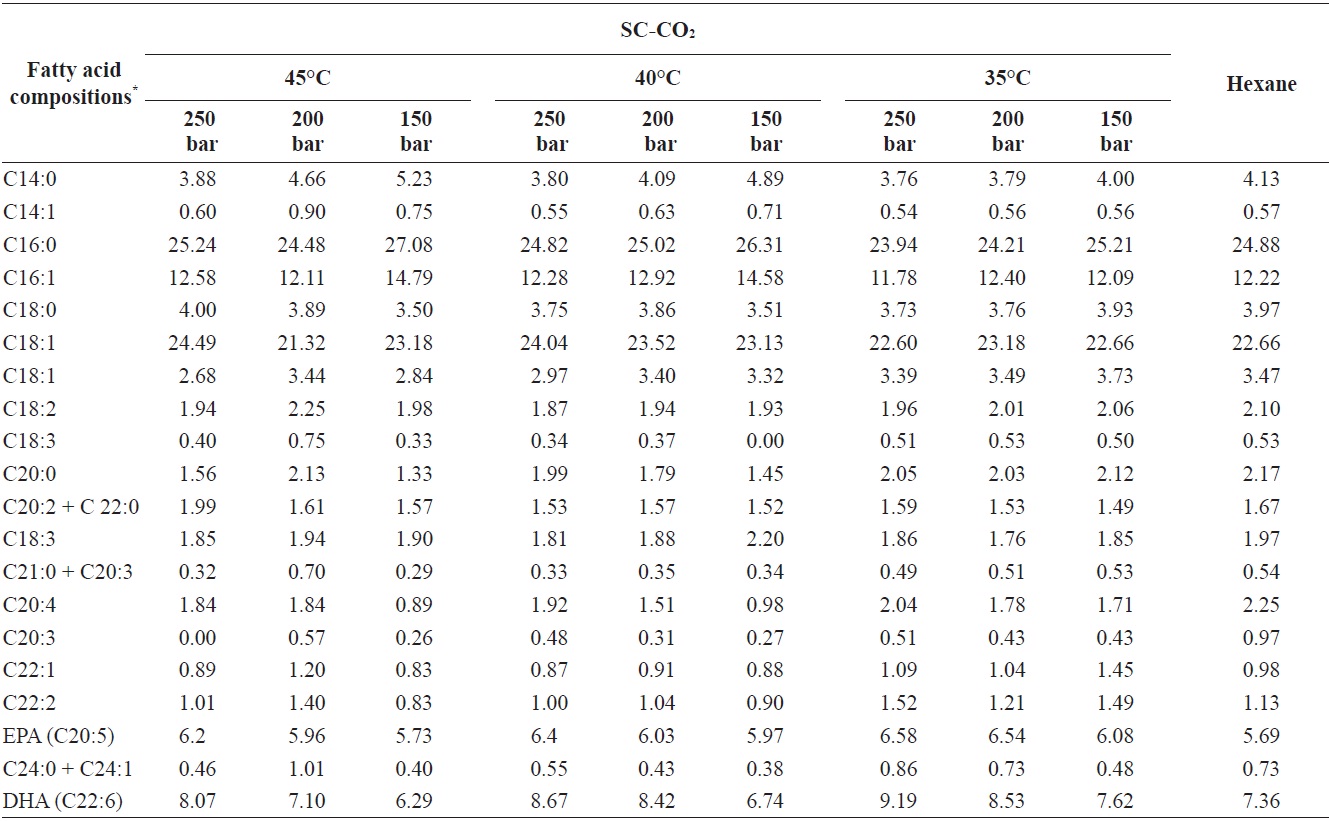
The fatty acid compositions of the oils obtained by SC-CO2 and hexane extraction without Soxhlet apparatus are shown in Table 1. In total, 20 fatty acids were identified in the extracts. The highest percentage of total fatty acids was identified
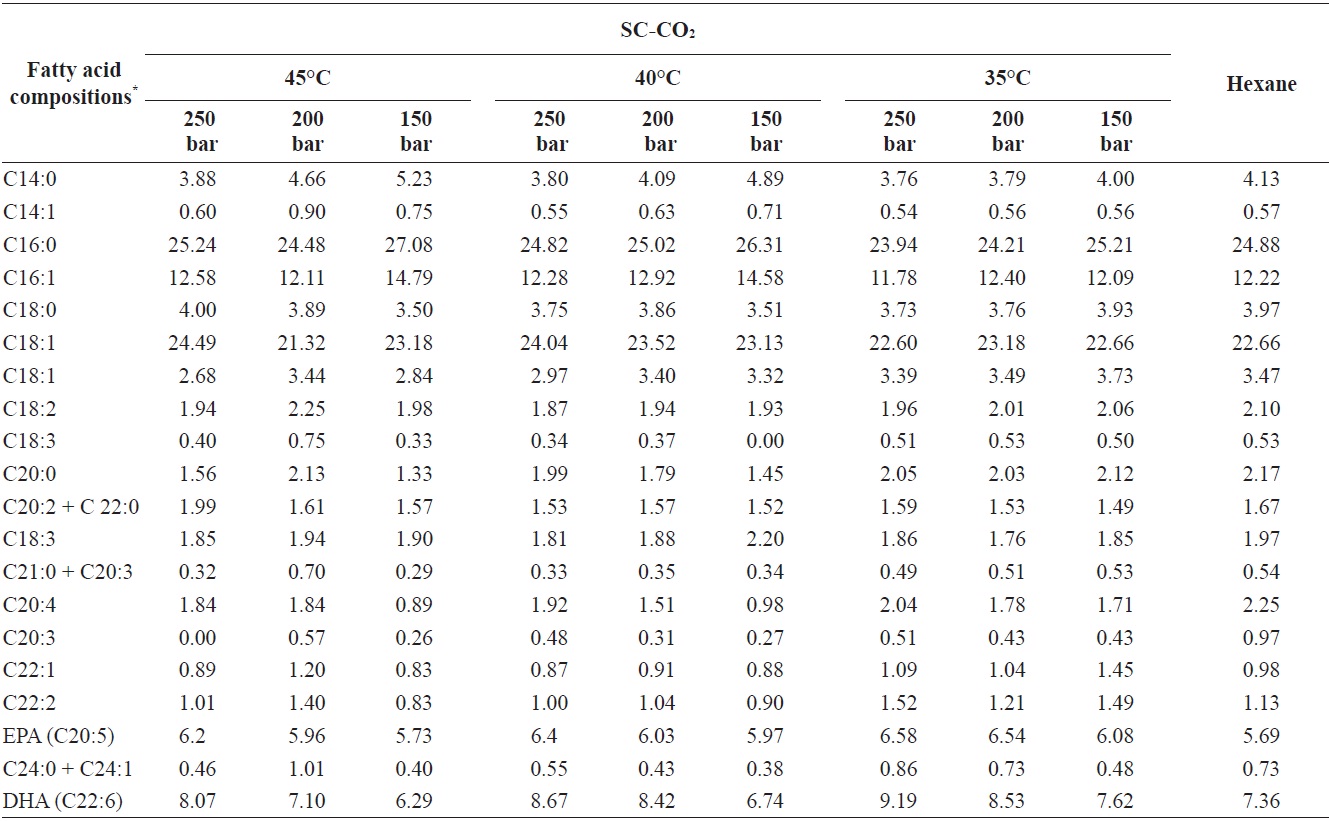
Fatty acid compositions percentage of yellow croakers muscle oil obtained by SC-CO2 and hexane extraction
at 250 bar and 35℃. Among saturated fatty acids, palmitic acid (C16:0) was present at the highest concentration 23.94% to 27.08% of the total identified fatty acids. Arachidic acid, which was found under all extraction conditions, is used to produce of detergents, photographic materials, and lubricants. Among monounsaturated fatty acids, oleic acid (C18:0) was also found in substantial amounts 21.32% to 24.49% of the total identified fatty acids. DHA (C22:6) was present in yellow croaker muscle oil at higher amounts than other PUFAs. The percentages of EPA (C20:5) and DHA (C22:6) in the total identified fatty acids ranged from 5.73% to 6.58% and 6.74% to 9.19%, respectively. The PUFA composition in yellow croaker muscle oil was identical to that in marine fish oils such as cod liver oil and anchovy oil, which contain about 14-31% of EPA and DHA (AOCS, 1997).
Additionally, the oil extracted using SC-CO2 had a higher percentage of PUFAs than the oil extracted using hexane, perhaps because of the high temperature and long extraction period used compared with SC-CO2 extraction. A long extraction period with heat may lead to the thermal degradation of fatty acids, especially unsaturated fatty acids. Similar results have been reported for the fatty acid profile of hake by-products (Rubio-Rodriguez et al., 2008)
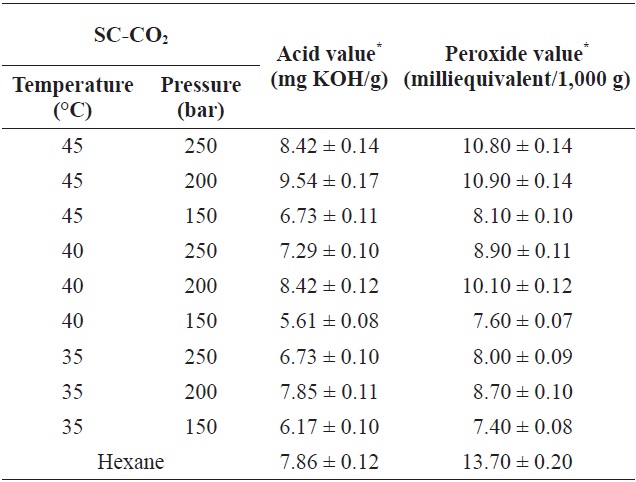
Marine fish oil contains high levels of PUFAs, and the speed at which oil deteriorates depends strongly on its production and storage conditions (Kamal-Eldin and Yanishlieva, 2002). The AV and POV of the oils extracted in this study using SC-CO2 and hexane are given in Table 2. The AV and POV were fairly high for hexane extracted oil compared to the SC-CO2 extracted oil. Also, the oil obtained at a higher extraction temperature using SC-CO2 had a higher AV and POV. The AV is used to represent the acidity of oil; a low AV indicates high oxidative stability (Essien et al., 2012). In contrast, the POV of an oil or fat is used as a measurement of rancidity, which occurs by autoxidation. Low exposure to oxygen during the SC-CO2 extraction process caused minimal oxidationnd POV of yellow croaker muscle oil under different SC-CO2 extraction conditions ranged from 5.61 ± 0.08 to 9.54 ± 0.17 mg KOH/g and 7.40 ± 0.08 to 10.90 ± 0.14 meq/1,000 g, respectively. The respective AV and POV of soybean oil were found to be 9.75% and 0.5 meq/g (Ashaye and Olusoji, 2006), which is higher than the values obtained for yellow croaker oil. However, the oil extracted using SC-CO2 showed higher stability compared to the oil obtained by hexane extraction without Soxhlet apparatus.

Acid value and peroxide value of yellow croaker muscle oil obtained by SC-CO2 and hexane extraction