The effects of inorganic mercury on hematological parameters and hepatic oxidative stress enzyme activity were studied in olive flounder Paralichthys olivaceus. Fish were injected twice intraperitoneally with mercuric chloride (2, 4, or 8 mg Hg/kg BW). The major hematological findings were significant decreases in the red blood cell count, hematocrit value, and hemoglobin level in olive flounder exposed to 8 mg Hg/kg BW. Remarkably low levels of calcium and chloride, and reduced osmolality, were also observed at 8 mg Hg/kg BW. In hepatic tissue, significant increases in glutathione peroxidase and catalase activity were observed above 4 mg Hg/kg BW Inorganic mercury also increased glutathione S-transferase and glutathione reductase activity at 8 mg Hg/kg BW in hepatic tissue. The present findings suggest that exposure to a low concentration (≥4 mg Hg/kg BW) of inorganic mercury can cause significant changes in hematological and antioxidant parameters.
Toxic heavy metals are increasingly being released into the environment as a result of industrialization. Mercury is a nonessential element that can have severe, toxic effects on aquatic animals when present in excessive amounts (Nriagu and Pacyna, 1988; Fitzgerald and Clarkson, 1991). Most of the mercury in water, sediments, or the biota is in the form of inorganic mercury salts or organic forms. Mercury has always been present at varying levels in environmental media and the biota, and all mercury is, in a sense, naturally occurring; that is, mercury is not a substance of human origin. Anthropogenic activities are thought to redistribute mercury from its original matrix through the atmosphere to other environmental media. Numerous studies have shown that the amount of mercury being deposited from the atmosphere has increased since the onset of the industrial age (Johansson et al., 1991; Nater and Grigal, 1992; Swain et al., 1992). Some mercury deposits arise from natural sources while others are derived from anthropogenic activities.
The absorption, distribution, metabolism, and excretion of mercury depend on its form and oxidation state (Goyer, 1991). Organic mercurials are more readily absorbed than inorganic forms. An oxidation-reduction cycle is involved in the metabolism of mercury and its compounds in animals, including humans. Mercury poisoning causes necrosis in epithelial cells, epithelial hyperplasia, and Na+-K+ ATPase inhibition in fishes (Bouquegneau, 1977; Lock et al., 1981). During early embryonic development in zebrafish, elevated mercury levels cause reduced survival time and increased hatching time (Dave and Xiu, 1991). The tissue distribution of inorganic mercury in fishes varies with the route of administration (water, oral, and intraperitoneal); however, the liver and kidneys tend to accumulate the highest quantity of this metal overall (Weisbart, 1973). Nevertheless, data on oxidative stress due to mercury exposure are lacking in fish.
Therefore, the objective of this study was to evaluate the effects of inorganic mercury delivered via an intraperitoneal injection on hematological parameters and hepatic antioxidant enzyme activity in olive flounder
Olive flounders
Mercury exposure took place in 0.5-ton fiberglass-reinforced plastic tanks containing 25 fish per treatment group. Each tank received a flow of 7 L min-1 with continuous aeration. Mercury(II) chloride (Sigma, St. Louis, MO, USA) was dissolved in phosphate-buffered saline (PBS) immediately before intraperitoneal injection. The fish were injected with 2, 4, or 8 mg Hg/kg BW as mercury chloride. The first injection was given 3 weeks after acclimatization; the second was given 1 week after the first treatment. The control group was subjected to the same regimen; however, they were injected with
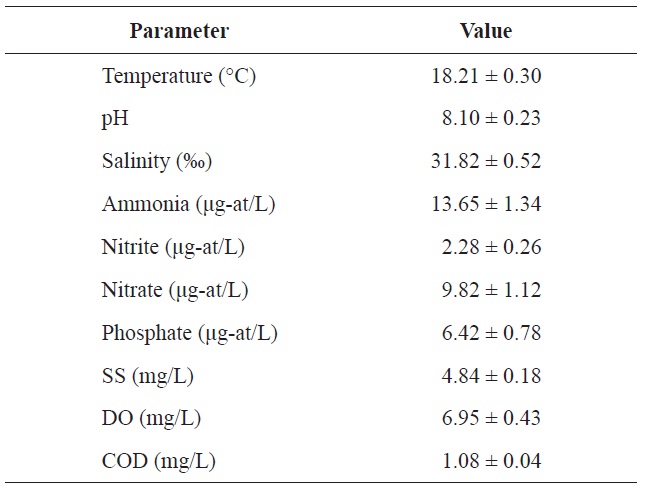
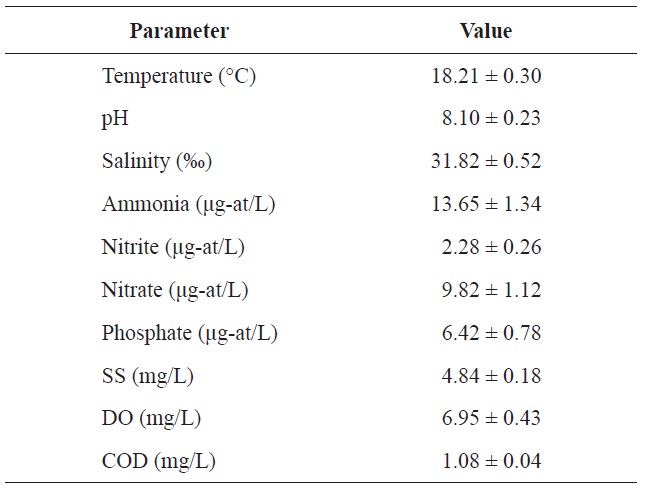
Chemical components of seawater used in the acclimation period of olive flounder Paralichthys olivaceus
an equal volume of PBS. Blood and hepatic tissue samples were taken to examine several hematological and antioxidant parameters at 1 and 2 weeks post injection.
>
Hematological parameter analysis
Blood samples were collected within 35-40 s through the caudal vein of the fish in 0.5-mL disposable heparinized syringes using a 30-gauge needle. The syringes were kept at 4℃ until the blood parameters were completely studied. The total red blood cell (RBC) count, hemoglobin (Hb) concentration, and hematocrit (Ht) value were determined immediately. The blood samples were centrifuged to separate erythrocytes from serum at 3,000
Liver tissues were excised and homogenized with 5 volumes of ice-cold homogenization buffer (50 mM Tris, pH 7.5, 1 mM EDTA, 1 mM DL-dithiothreitol, and 150 mM NaCl) with several strokes using a Teflon pestle (099CK4424; Glas- Col, Terre Haute, IN, USA). The homogenate was centrifuged at 12,000
Statistical analyses were performed using the SPSS/PC+ statistical package (SPSS Inc., Chicago, IL, USA). Significant differences between groups were identified using one-way ANOVA and Duncan’s test for multiple comparisons or Student’s
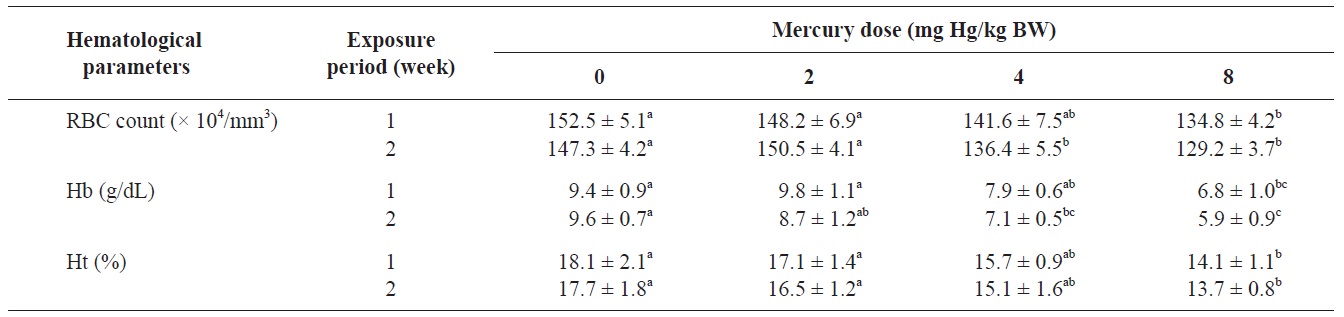
The RBC count, Hb concentration, and Ht value of olive flounders exposed to different levels of inorganic mercury are summarized in Table 2. The major hematological findings were a significant decrease in the RBC count and Hb concentration in olive flounders exposed to >4 mg Hg/kg BW compared with the control group after 2 weeks (
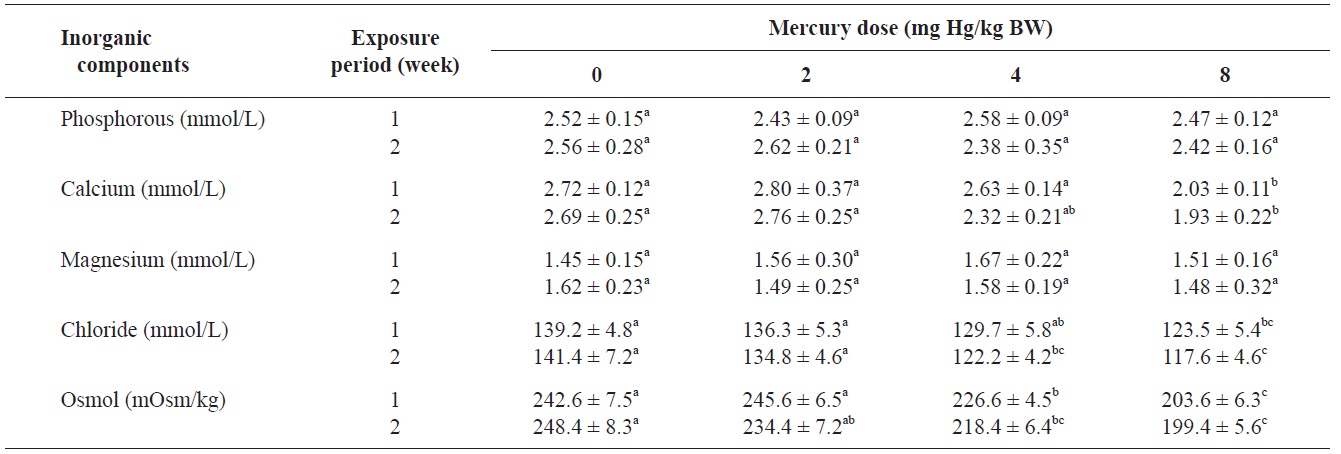
The blood serum inorganic components of olive flounder treated with inorganic mercury are shown in Table 3. No significant differences were observed in serum phosphorous or
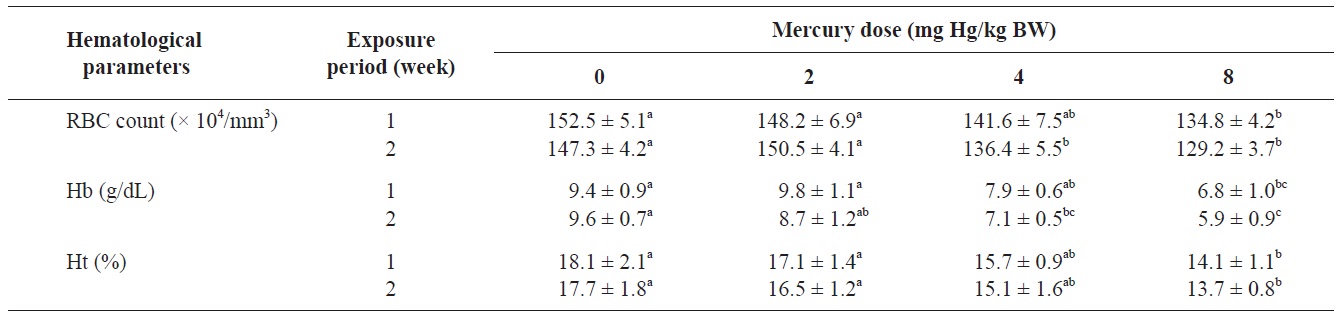
Hematological parameters of olive flounder Paralichthys olivaceus exposed to the various inorganic mercury concentrations
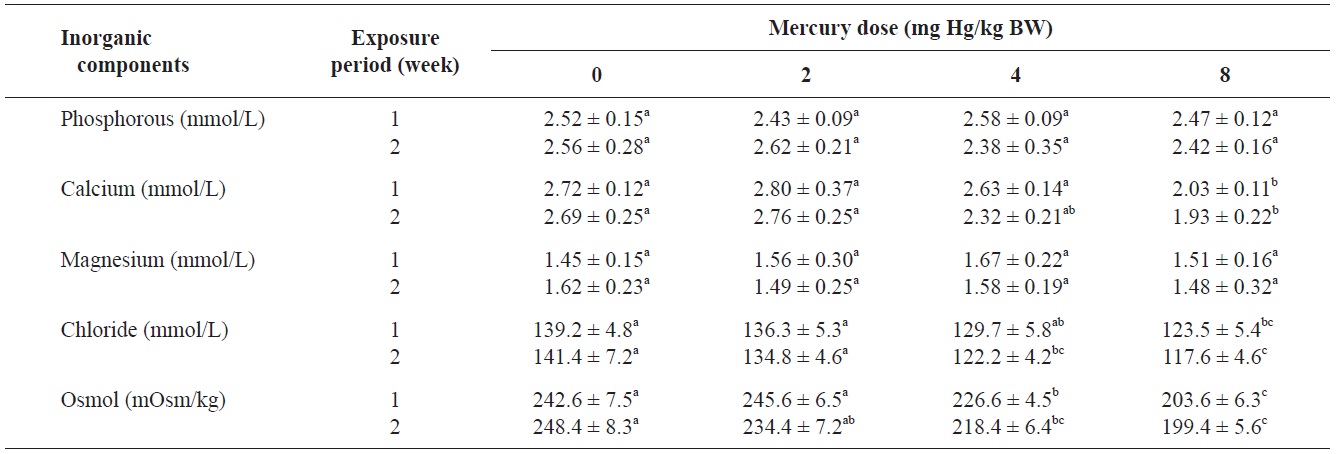
Inorganic components and osmolality of olive flounder Paralichthys olivaceus exposed to various inorganic mercury concentrations
magnesium among the treatment groups. However, a clear decreasing trend was noted in serum calcium and chloride at 8 and ≥4 mg Hg/kg BW, respectively, compared to the controls after 2 weeks (P<0.05). The control group maintained a normal blood osmolality (between 242 and 248 mOsm/kg). However, at 1 and 2 weeks, the blood osmolality in the fish exposed to >4 mg Hg/kg BW had significantly decreased compared to the controls (
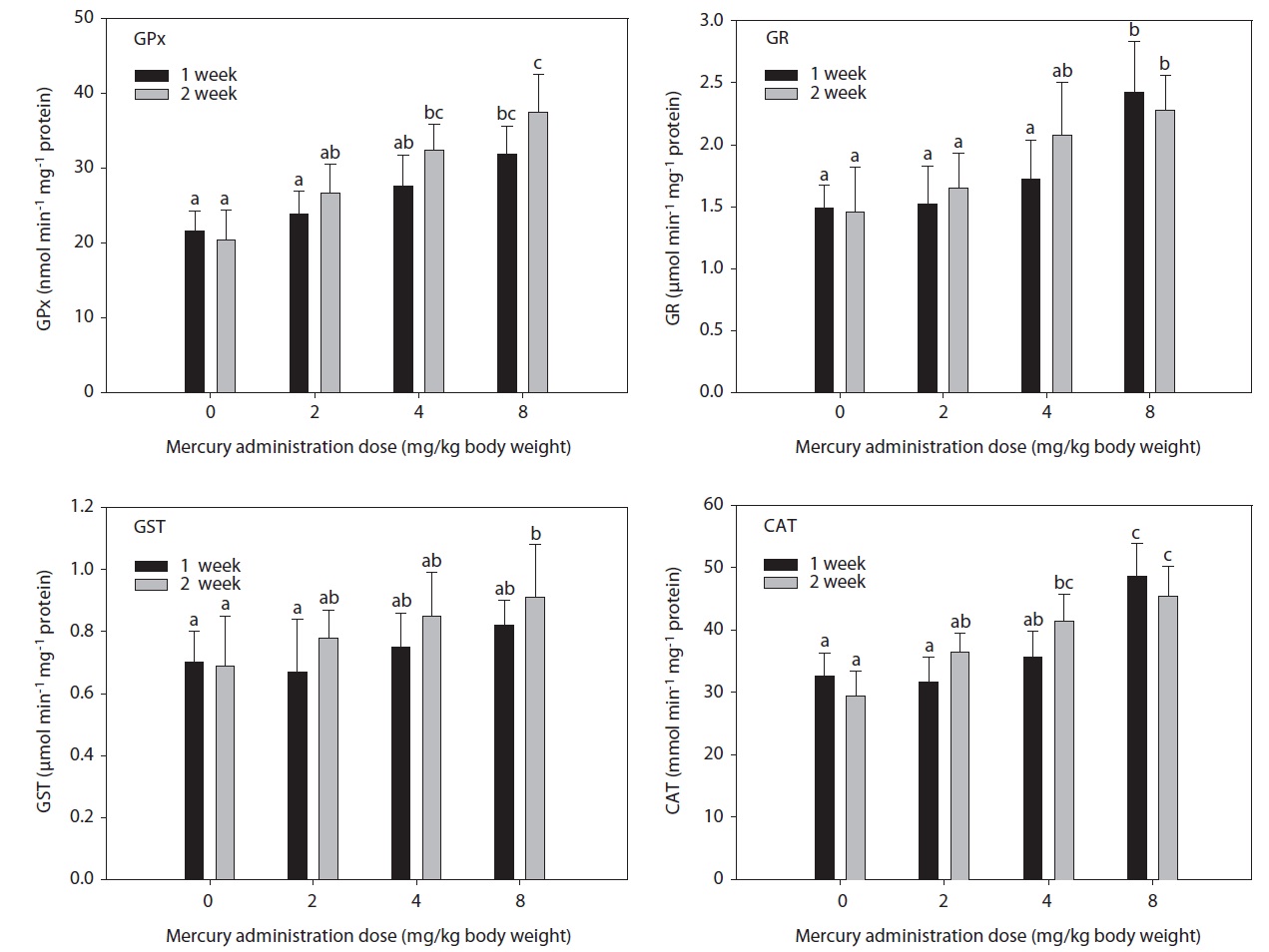
The hepatic GPx, GST, GR, and CAT activity levels in fish exposed to inorganic mercury are presented in Fig. 1. GPx, GST, GR, and CAT activity in olive flounders treated with inorganic mercury at a concentration of >2 mg Hg/kg BW increased in a dose- and time-dependent manner. GPx and CAT activity in olive flounders exposed to inorganic mercury at concentrations ≥4 mg Hg/kg BW increased significantly compared to the controls after 2 weeks (
in GST activity was observed at 8 mg Hg/kg BW after 2 weeks (
The blood properties of fish are suitable biomarkers for evaluating the potential risk of chemicals (Roche and Boge, 1996). Past investigators have identified changes in several hematological variables as indicators of heavy metal exposure (Cyriac et al., 1989). The predominant hematological findings in this study were a significant decrease in the RBC count and Hb concentration in olive flounders exposed to inorganic mercury at >4 mg Hg/kg BW. The Ht value in fish following inorganic mercury exposure to 8 mg Hg/kg BW for 2 weeks also decreased. Chronic exposure of fish to heavy metals causes serve reductions in the RBC count, Ht, and Hb concentration (Tewari et al., 1987). Mercury has been shown to cause anemia in fish by inhibiting Hb synthesis and shortening the life span of circulating erythrocytes (Houston et al., 1993). These results are in accordance with those of mercury-exposed
No significant differences in serum phosphorous or magnesium were detected among the treatment groups. However, a significant decrease in calcium and chloride was observed at 8 and ≥4 mg Hg/kg BW, respectively, at 2 weeks. Previous laboratory studies have documented the inhibitory effects of various metals on gill function in fish (Evans, 1987; Watson and Benson, 1987). Indeed, the gills of freshwater teleosts function as the primary site for the active absorption of ions from the external medium and for the exchange of respiratory gases. Mercury can cause altered osmoregulation in both marine and freshwater fish. In this study, the blood osmolality of olive flounder exposed to >4 mg Hg/kg BW was significantly decreased compared to the controls. This result can be explained by direct mercury-induced osmoregulation failure. Lock et al. (1981) suggested that mercury caused osmoregulatory effects primarily by increasing gill permeability to water. Stinson and Mallatt (1989) reported increased permeability of the gills in lamprey in response to mercury poisoning.
The main enzymes that detoxify reactive oxygen species in all organisms are GPx (EC 1.11.1.9), peroxidase (EC 1.11.1.7), and CAT (EC 1.11.1.6), all of which are abundant in fish tissue (Di Giulio et al., 1993). These enzymes, which are induced by reactive oxygen species, may be useful indicators of oxidative stress. The phase II system and endogenous cellular glutathione have received relatively little attention as environmental indicators (Stein et al., 1992). The induction of antioxidants is a sensitive early warning signal of incipient oxidative stress (Benson and Di Giulio, 1992). GST has been identified in all organisms in which it has been investigated (Diericx, 1984; Stenersen et al., 1987), and it seems likely that it is ubiquitous. GST activity is known to increase in rats exposed to polychlorinated biphenyls (Kamohara et al., 1984). The present findings corroborate the observations of Davies (1985) and Fair (1986) in fish exposed to chlorothalonil and benzo(a)pyrene, respectively.
Similarly, in a previous study, cadmium exposure in rainbow trout for 4 weeks caused an initial decrease followed by a net increase in hepatic GST (Forlin et al., 1986). In this study, hepatic GST activity in olive flounders toward CDNB after 2 weeks of exposure to 8 mg Hg/kg BW was markedly elevated. These results demonstrate that GST activity was significantly altered by treatment with inorganic mercury. The status of other antioxidant systems is variably affected by polycyclic aromatic hydrocarbon (PAH) exposure (Niyogi et al., 2001; Veignie et al., 2004).
In this study, hepatic GR activity was significantly higher in fish exposed to 8 mg Hg/kg BW after 1 week compared to the controls. Similar results were reported by Stephensen et al. (2003), who observed increased hepatic GR activity in fish exposed to PAHs under laboratory conditions as well as increased GR activity in fish inhabiting a PAH-polluted harbor. GPx is the most important peroxidase for the detoxification of hydroperoxides. It catalyzes the glutathione-dependent reduction of hydroperoxides and hydrogen peroxide (H2O2). GPx activity may be induced by environmental pollutants. Its activity increased together with GR in rainbow trout injected with tetrachlorobiphenyl (Otto and Moon, 1995) and carp exposed to copper, but not to paraquat (Matkovics et al., 1987). In this study, hepatic GPx and CAT activity in olive flounders exposed to inorganic mercury at ≥4 mg Hg/kg BW was significantly elevated compared to the controls after 2 weeks. GPx has been postulated to protect erythrocytes from damage by H2O2 and to be responsible for the reduction of lipid hydroperoxides. Therefore, it is hypothesized that this enzyme protects tissue against oxidative damage due to lipid peroxidation. The liver is a major site of detoxification and the first target of ingested oxidants; thus, it is considered to be an important tissue in the study of the protective role of GPx in lipid peroxidation. CAT occurs primarily in peroxisomes. Its activity increased together with other peroxisomal enzymes in fish liver upon exposure to bleached kraft mill effluents, suggesting the induction of peroxisome proliferation (Mather-Mihaich and Di Giulio, 1991). Some pollutants may inhibit CAT activity. High concentrations of copper were shown to inhibit CAT activity in liver, gill, and muscle; a similar effect was induced by 100 ppm of ZnSO4 in gill and muscle (Radi and Matkovics, 1988).
We conclude that exposure to a low concentration (≥4 mg Hg/kg BW) of inorganic mercury resulted in significant changes in several hematological and antioxidant parameters.