Rotifers are a small type of zooplankton; however, in the world of aquaculture, rotifers are a very large object of attention by scientists and aquaculturists. The rotifer has been used for the seedling production of many economically important marine organisms, as it can be cultured at high densities. However, a very important unsolved problem remains: these cultures sometimes “crash” as characterized by a rapid and sudden population decrease in mass rotifer culture tanks. The cause of this phenomenon is not well known.
Marine rotifers of the genus Brachionus are widely used as live food for rearing early-stage larval marine fish. In mass cultures of rotifers, contamination by various organisms is commonly observed, and many of these contaminants can affect rotifer growth, e.g., copepods, protozoans, and anostracans (Hagiwara et al., 1995; Jung et al., 1997). Maeda and Hino (1991) examined the roles of contaminant protozoan species in rotifer mass-culture tanks. Reguera (1984) reported that a harmful ciliate in isolated rotifer mass-culture tanks reduced the population growth of the rotifer B. plicatilis and the alga Dunaliella (rotifer food) through food competition. Jung et al. (1997) reported that other protozoan species, with the exception of Vorticella sp., also reduced rotifer population growth.
The objectives of the present study were to artificially control the density of the ciliates Euplotes sp. and Vorticella sp. via competition in rotifer microcosms and to apply this bio-control method to the stable production of the rotifer Brachionus rotundiformis by adding Vorticella sp. to cultures. By applying tripartite-specific relationships and incorporating Euplotes sp. and Vorticella sp. into B. rotundiformis cultures, I aimed to develop more stable rotifer culturing techniques.
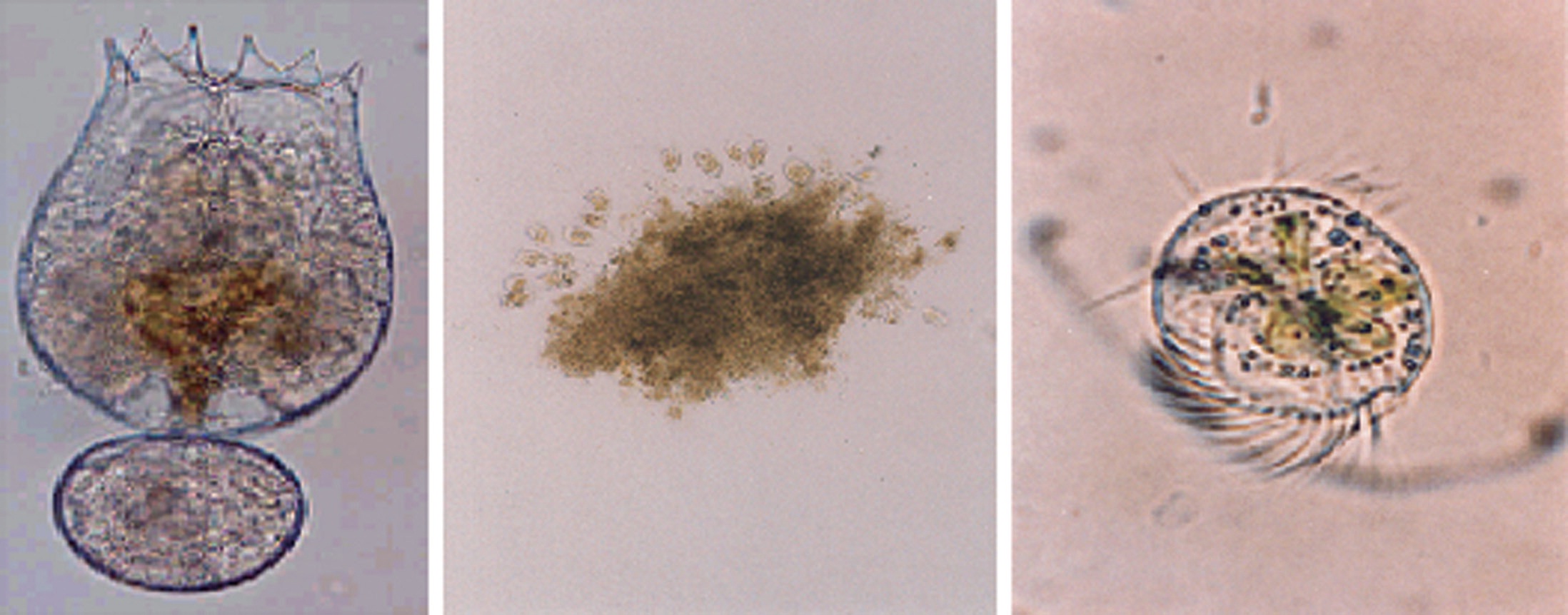
The test rotifer was B. rotundiformis of the Koshiki strain (Fig. 1A). The two most frequently observed contaminant protozoan species, Vorticella sp. (Fig. 1B) and Euplotes sp. (Fig. 1C), were isolated from rotifer mass-culture tanks. After collection, these organisms were maintained as monocultures in the laboratory. Culture conditions were 22 ppt salinity and 25℃ temperature. Food (centrifuged Nannochloropsis oculata) for the test organisms was maintained daily at about 7 × 105 cells/mL during the experimental period. N. oculata was cultured using modified Erd-Schreiber medium in an indoor culture room (Hagiwara et al., 1994). The genus of both test protozoans was identified in pure mono-species cultures (Inoki, 1981).
The study design, which focused on either the interspecific relationship between the rotifer and a protozoan (Euplotes sp. or Vorticella sp.) or the tripartite-specific relationship of the rotifer and both protozoans (Euplotes sp. and Vorticella sp.) species, was based on the methods of Jung et al. (1997). I examined the status of the coexistence of the two contaminating protozoans as well as on interspecific interactions between the two and the effect thereof on rotifer population growth.
Three treatments were implemented: 1) rotifers in singlespecies culture (SR); 2) two types of two-species mixed cultures (B. rotundiformis and Euplotes sp., SR + E; B. rotundiformis and Vorticella sp., SR + V); and 3) two types of tripartite mixed cultures (Vorticella sp. added at the start of cultures, SR + E + V; Vorticella sp. added 6 days after the start of cultures, SR + E/V).
Culture volumes of the small-scale experiment were 40-mL triplicates in 50-mL glass beakers (Pyrex). All beakers were covered with parafilm (American Can Co., Greenwich, CT, USA). Semi-mass cultures were maintained in 30-L polycarbonate round tanks with 5 L of culture water. Two types of culture water were used in the experiments: sterilized sea water diluted with distilled water for the small-scale experiment and natural sea water diluted with tap water for semi-mass cultures. In the 5-L semi-mass cultures, the formula for natural culture water (natural sea water diluted with tap water) followed the culture methods used in mass culture at sea farming centers.
The initial number of organisms was 20 amictic female B. rotundiformis and each five cells of the two protozoans, Euplotes sp. and Vorticella sp., per 40 mL for both experimental designs (small-scale 40-mL cultures and 5-L semi-mass cultures).
Both experiments were maintained in total darkness, except for observation times. The experimental culture period was 30 days. Every 6 days, the number of test organisms in three, 4-mL sub-samples per culture tank was counted for both experiments. After counting, all zooplankton species were returned to the experimental culture tanks.
Results were analyzed using t-tests with a 95% confidence level.
No predator-prey interactions were observed between the rotifer, B. rotundiformis (190 ± 20 μm) (Fig. 1A) and the two ciliates, Vorticella sp. (70 ± 13 μm) (Fig. 1B) and Euplotes sp. (82 ± 16 μm) (Fig. 1C) or between the two experimental ciliates.
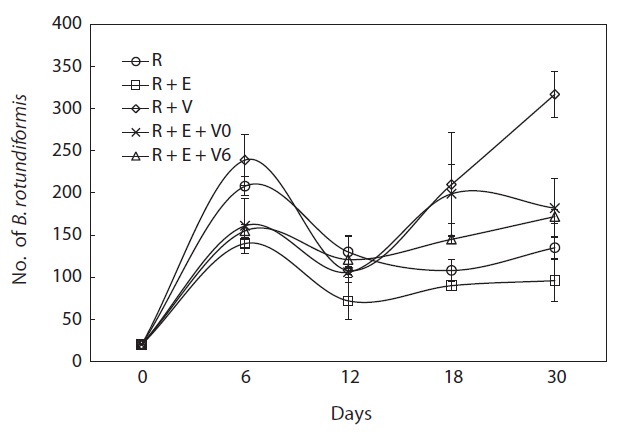
In the small-scale 40-mL cultures, the growth of rotifers was clearly suppressed when Euplotes sp. was present (SR + E) (Fig. 2) compared with the ciliate-free rotifer cultures (SR) (Fig. 2). However, the addition of Vorticella sp. (SR + V) increased rotifer growth by 2.1 times compared with that observed in the Euplotes sp. contaminated cultures (P < 0.01) (Fig. 2). In two of the three-species tripartite mixed cultures (SR + E/V and SR + E + V), no declines in rotifer population growth were observed relative to the ciliate-free rotifer culture or to the mixed cultures of rotifers and Euplotes sp. (P < 0.05) (Fig. 2).
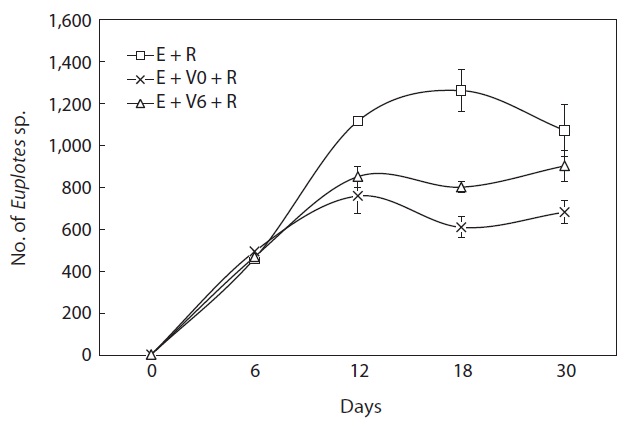
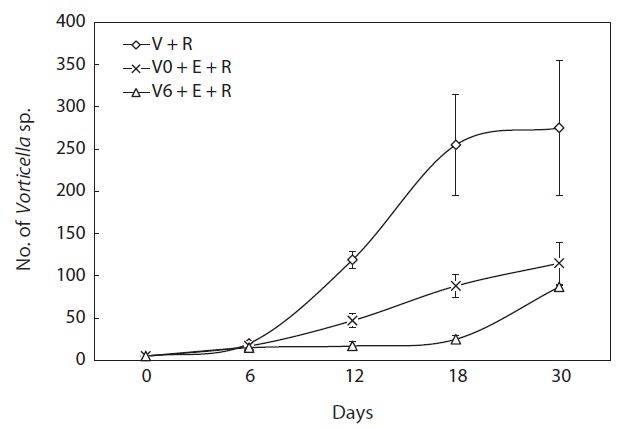
The population growth of each ciliate, i.e., Euplotes sp. and Vorticella sp., interfered with that of the other (Figs. 3,4). The density of Euplotes sp. rapidly increased in cultures with only the rotifer compared with conditions of co-existence with Vorticella sp. Additionally, Vorticella sp. grew quickly in mixed cultures with only the rotifer compared with tripartite mixed cultures including Euplotes sp. (Fig. 4).
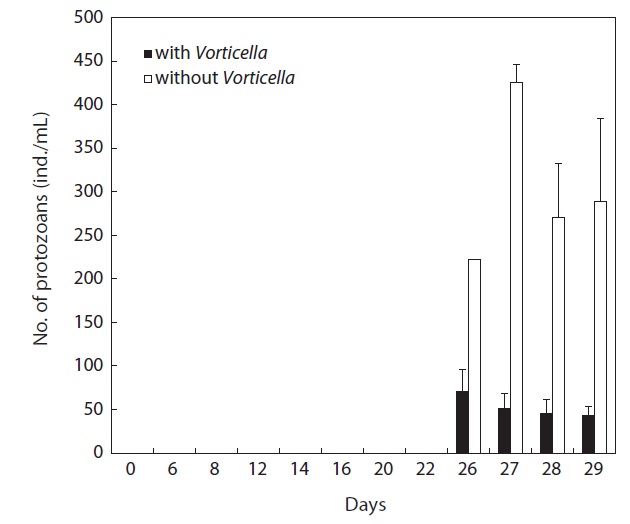
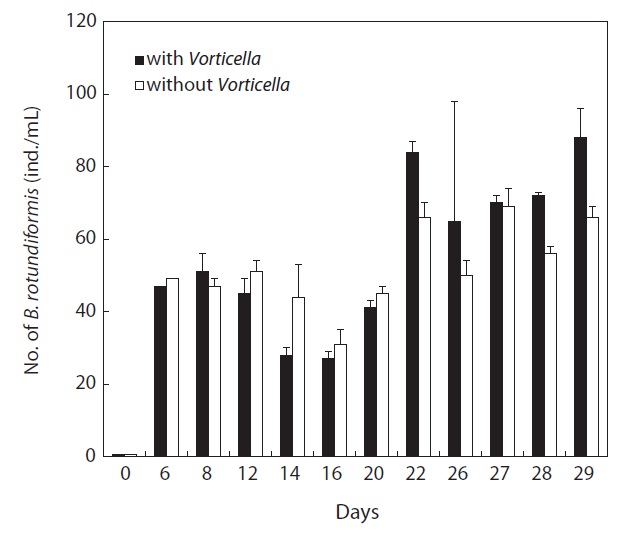
When Vorticella sp. was artificially added to the 5-L semimass culture tanks of rotifers, this ciliate heavily suppressed the growth of the contaminating protozoan species (Euplotes sp.) by 0.1 times compared with Vorticella sp.-free cultures (P < 0.01) (Fig. 5). In contrast, rotifer population growth was not suppressed and was even marginally increased by 1.1 times the growth observed in ciliate-free rotifer cultures (Fig. 6).
The present study examined the interspecific relationships between two zooplankton species within microcosms. Moreover, the micro-ecological tripartite-specific relationships of three zooplankton species were also investigated. Previous studies have examined similar multi-species relationships within microcosms. For example, Kawabata et al. (1995) achieved long-term cultures of species-defined microcosms using Escherichia coli DH5a as a decomposer bacteria, Tetrahymena thermophila B as a consumer protozoa, and Euglena gracilis Z as the producer algae under artificial culture medium conditions. This artificial small microcosm was successfully maintained as a stable ecological system in a micro-community (Kawabata et al., 1995).
The present results demonstrate the potential for co-existence of as well as the interspecific relationships between two contaminating protozoan ciliate species (Euplotes sp. and Vorticella sp.). These interspecific interactions directly or indirectly affected the population growth of a rotifer species, B. rotundiformis, within the same culture community. Pace and Orcutt (1981) also examined the function of protozoans in living environmental communities, demonstrating that a population of crustaceans declined but those of protozoans and a rotifer increased when cultured together. Pierce and Turner (1992) documented the importance of marine planktonic ciliates as consumers within a tropical link system and as a vector
of energy flow through pelagic marine ecosystems. Hamilton and Preslan (1969) examined the optimal conditions of ciliate growth in terms of feeding behavior and food (bacteria) concentration.
During the experiments, no predator-prey interactions were observed between the rotifer (190 ± 20 μm) (Fig. 1A) and either of the two test ciliates, Vorticella sp. (70 ± 13 μm) (Fig. 1B) and Euplotes sp. (82 ± 16 μm) (Fig. 1C). In contrast, Gilbert and Jack (1993) showed that Synchaeta pectinata (320 μm), Brachionus calyciflorus (280 μm), and Asplanchna girodi (491 μm) were effective predators of ciliates (45-60 μm), ingesting up to 50 ciliates rotifer-1 day-1.
The common contaminating ciliate, Euplotes sp., always interferes with the stability of rotifer mass cultures in culture tanks (Jung et al., 1997, 2008). Jung et al. (1997) showed that all contaminating ciliates, except for Vorticella sp., suppressed rotifer population growth. The present results indicate that one method for suppressing Euplotes sp. populations within cultures is to utilize the bacterivorous ciliate Vorticella sp. to induce food (bacteria) competition between Euplotes sp. and Vorticella sp. Generally, ciliate groups feed on bacteria. As a result, rotifer population growth is directly and indirectly improved due to declines in harmful Euplotes sp. caused by food (aquatic bacteria) competition with Vorticella sp. Euplotes sp. competes for the same food (centrifuged and condensed instant microalgae) as the marine Brachionus rotifer within the same culture tanks (Jung et al., 2008). Furthermore, an isolated bacteria group (Flavobacterium) in Euplotes-contaminated rotifer culture tanks can also strongly suppress rotifer growth (Maeda and Hino, 1991). Thus, Vorticella may experience rapid growth in Euplotes-contaminated tanks by utilizing these bacteria as a food source.
The present study presents a very interesting phase of rotifer
microcosms. In the experimental microcosms, intricate relationships between two protozoans, namely inter- and tripartite-specific interactions, caused direct and indirect effects on rotifer population growth. Moreover, the Vorticella sp. contaminant was very useful as an aquatic ecosystem control tool. These results demonstrate the value of Vorticella sp. for maintaining the stable production of rotifer mass cultures.