The class Cephalopoda is composed of two extant subclasses, Nautiloidea (Nautilus and Allonautilus) and Coleoidea. The Coleoidea subclass also contains two subdivisions, the Belemnoidea, which became extinct at the end of the Cretaceous period, and the Neocoleoidea, which contains octopuses, squids, and cuttlefishes (Strugnell and Nishiguchi, 2007). Octopuses, squids, and cuttlefishes are widely consumed in Korea, where they are appreciated for their taste and texture as well as health-functional constituents, such as taurine and collagen, and nutritional components, such as amino acids, minerals, and vitamins (Kim et al., 1997). Industries in Korea process octopus, squid, and cuttlefish into diverse products, including dried, seasoned, salted, smoked, frozen, boiled, and pureed (“surimi”) products (Ryu et al., 1992; Choi et al., 1995).
More than 40% of the total body weight is estimated to end up as processing by-products. Typical unused portions are the viscera, skin, boiling water, and cuttlebone. Such by-products of the Neocoleoidea are often used to produce fish meals and fertilizers or are directly discharged into estuaries, resulting in environmental pollution and offensive odors. However, the Neocoleoidea processing by-products might be useful in other food products, as they contain high amounts of useful food components, such as eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), enzymes, and protein in the viscera, collagen and collagen-derived peptides in the skin, taste components in the boiling water, chitin in the pen shell, and minerals in cuttlebone (Lian et al., 2005). Notably, the hepatopancreas (HP) accounts for 14 to 20% of a Neocoleoidea’s body weight and contains 14 to 15% crude protein (Sugiyama et al., 1989) and various proteolytic enzymes with high activity (Raksakulthai and Haard, 1999; Kishimura et al., 2001; Ezquerra-Brauer et al., 2002).
Food products may benefit from treatment with proteolytic enzymes that improve palatability and functional properties of the protein. Proteolytic enzymes are commonly used as processing aids for bread, beer, cheese, and fish sauce (Raksakulthai and Haard, 2001). The enzymes from the HP of the Neocoleoidea may serve as an excellent resource for extracting proteolytic enzymes, and it is thus important to characterize these enzymes in detail.
Previous studies have examined the Neocoleoidea HP as a resource for enzyme extraction. Subjects examined include the influence of the harvest season on the proteolytic activity of HP from the jumbo squid
Frozen HPs were obtained from the octopus
L-leucine-
Following methods of the Association of Official Analytical Chemists (AOAC, 1995), moisture was quantified by oven- drying at 105℃, total lipid levels by Soxhlet extraction, crude protein levels by the Kjeldahl procedure, and crude ash levels by incineration in a muffle furnace at 550℃.
>
Enzyme (endoprotease and exopeptidase) activity
The protein concentrations of crude extracts (CEs) from Neocoleoidea HPs were measured according to the method of Lowry (Lowry et al., 1951) using bovine serum albumin as the standard protein.
To extract CEs from Neocoleoidea HPs, the frozen Neocoleoidea HPs were partially thawed and homogenized in three volumes of deionized water. For enzyme activation, the homogenates were incubated at 20℃ for 4 h and then centrifuged at 12,000 g for 30 min at 4℃. The CE was obtained after the supernatant was treated with 0.2 volumes of carbon tetrachloride to remove lipids and centrifuged at 12,000 g for 30 min at 4℃.
Endoprotease activity toward azocasein was assayed by the method of Starky (1977) with modifications. CE from Neocoleoidea HP (20?200 μL) was mixed with 0.5 mL of 1% azocasein in 1.5 mL of 0.1 M sodium phosphate (pH 6.0 and 9.0) before incubation at 40℃ for 1 h. The reaction was stopped by the addition of 2 mL of 5% trichloroacetic acid (TCA) solution. The mixture was then centrifuged at 146
Exopeptidase activity was determined using a modified version of methods reported by Garcia-Carreno and Haard (1993). CE from Neocoleoidea HP (20?200 μL) was mixed with 0.04 mL of 0.5 mM LeuPNA in 1 mL of 0.1 M sodium phosphate (pH 6.0 and 9.0) before incubation at 40℃ for 1 h. The reaction was stopped by the addition of 0.2 mL of 33% acetic acid solution. The mixture was then centrifuged at 146 g for 15 min. The absorbance of the supernatant at 410 nm was measured and was considered to express the exopeptidase activity of the sample.
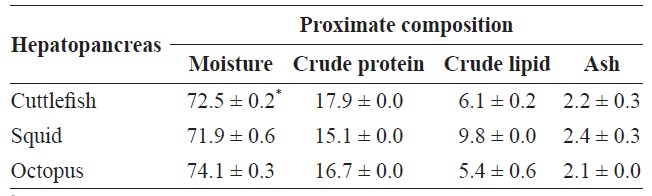
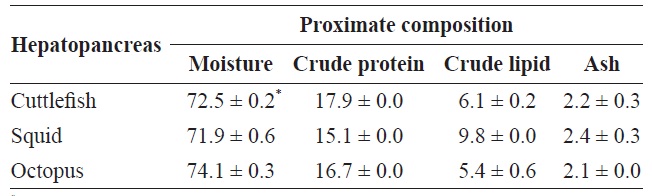
Proximate compositions of hepatopancreas from Sepia officinalis, Todarodes pacificus, Octopus vulgaris Cuvier.
One unit of enzyme activity (1 U/mg) was defined as the amount of endoprotease and exopeptidase required to change 0.1 digit per 1 h at 40℃ and pH 6 or 9. Specific enzyme activity was expressed as U/mg of protein. Total activities (U) of endoproteases and exopeptidases were calculated as total volume (mL) × protein concentration (mg/mL) × specific activity (U/mg).
>
Proximate composition of Neocoleoidea HP
Proximate compositions of the octopus, squid, and cuttlefish HPs are shown in Table 1. The proximate composition of the octopus HP was 74.1% moisture, 16.7% crude protein, 5.4% crude lipid, and 2.1% ash. The crude protein content (containing enzymes) of octopus HP was higher than that of squid HP (15.1%), but lower than that of cuttlefish HP (17.9%). The crude lipid content of octopus HP was lower than those of squid HP (9.8%) and cuttlefish HP (6.1%). The crude protein and crude lipid contents of Neocoleoidea HPs suggested that the highest yield of CE containing enzymes would be obtained from cuttlefish HP, followed by octopus HP and squid HP. Kim et al. (2007) reported that the proximate composition of the viscera of the Argentine shortfin squid (
>
Protein concentrations of the CEs
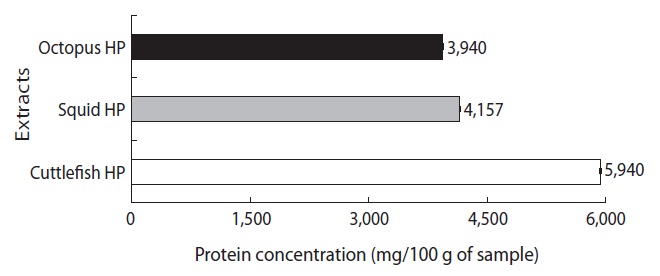
The protein concentrations of the CEs from the octopus, squid, and cuttlefish HPs are shown in Fig. 1. The protein concentration of the CE from octopus HP was 3,940 mg/100 g of HP, which was lower than values for the CEs from squid HP (4,157 mg/100 g of HP) and cuttlefish HP (5,940 mg/100 g of HP). These results suggest that the CE yields from Neocoleoidea HPs are highest from cuttlefish, followed by squid and octopus.
>
Enzyme activities of the CEs
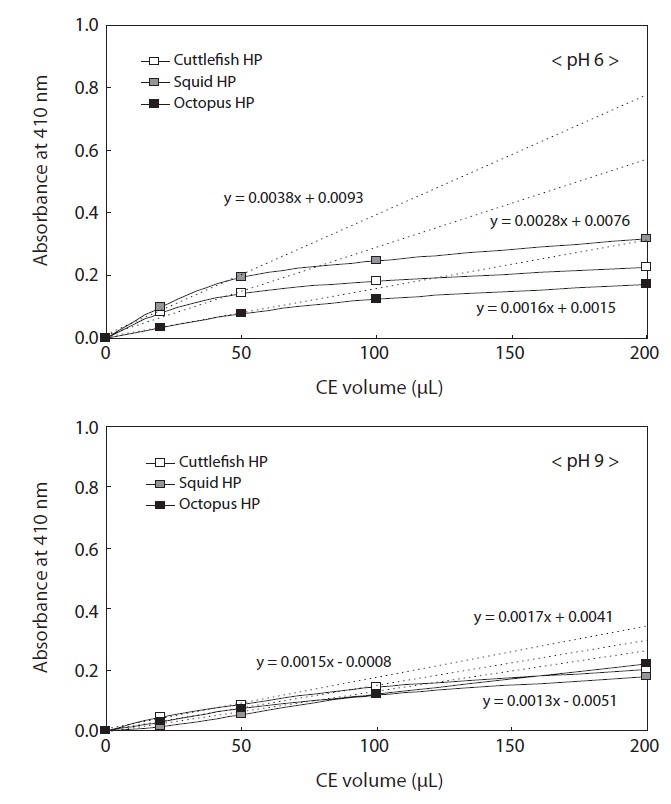
The protein concentrations of the CEs used to investigate enzyme activities were 19.80 mg/mL for cuttlefish HP, 15.59 mg/mL for squid HP, and 14.24 mg/mL for octopus HP. To investigate the endoprotease activities of the Neocoleoidea HP CEs, the effects of CE volume (0?200 μL) and pH of azocasein (6 and 9) on endoprotease activities were examined for the three studied species (Fig. 2). Regardless of the CE volume, azocaseinolytic activity, expressed as absorbance at 410 nm, toward azocasein at pH 6 was highest in the squid HP CE, followed by the cuttlefish and octopus HP CEs for the same volumes of CE. The azocaseinolytic activities of all the CEs increased rapidly and linearly with increasing extract volume up to a volume of 50 μL, after which they slowly increased. These results suggest that the optimum CE volume for the measuring azocaseinolytic activity of endoproteases toward azocasein at pH 9.0 is 50 μL for all the CEs. The azocaseinolytic activities of the regions with linear increases (0?50 μL) were expressed as the following linear functions: y = 0.0038x + 0.0093 for the CE from squid HP, y = 0.0028x + 0.0076 for the CE from cuttlefish HP, and y = 0.0016x + 0.0015 for the CE from octopus HP.
The azocaseinolytic activities of all the CEs from Neocoleoidea HPs toward azocasein at pH 9 also increased rapidly and linearly with increasing CE volume up to 50 μL, after which they increased slowly. These results suggest that the optimum CE volume for measuring azocaseinolytic activity of endoproteases toward azocasein at pH 9 is 50 μL for all the CEs. The azocaseinolytic activities of the regions with linear increases (0?50 μL) were expressed as the following linear functions: y = 0.0017x + 0.0041 for the CE from squid HP, y = 0.0015x - 0.0008 for the CE from cuttlefish HP, and y = 0.0013x - 0.0051 for the CE from octopus HP. Except for 200 μL, the azocaseinolytic activities of endoproteases in all the CE volumes toward azocasein at pH 9.0 were highest in the CE from cuttlefish HP, followed by the CE from octopus HP and the CE from squid HP.
The azocaseinolytic activities in all the CEs toward azocasein were markedly higher at pH 6 than at pH 9, suggesting distinct proteolytic enzyme activities in most samples in the weak acid to alkali pH range.
Kim et al. (2008a) reported that the endoprotease activities of a CE from
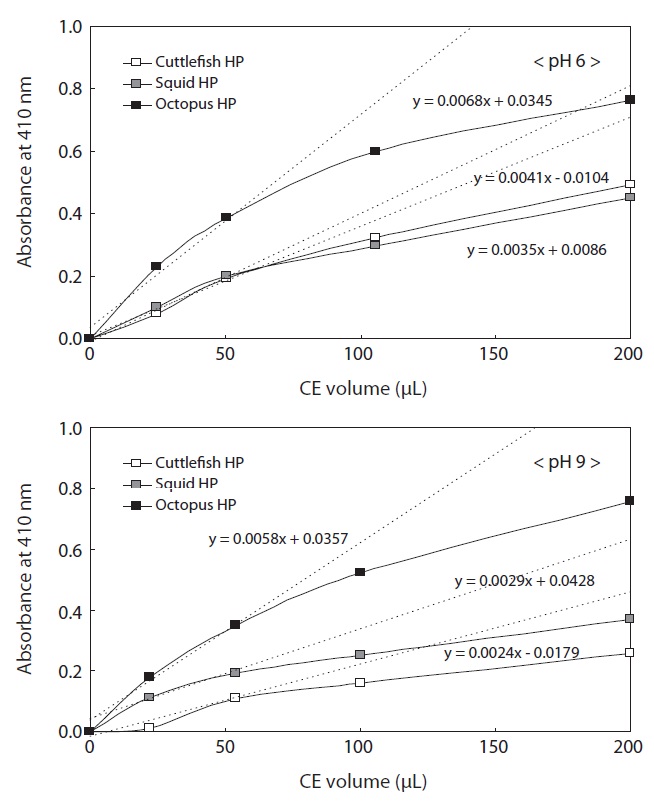
To investigate the exopeptidase activities in CEs from Neocoleoidea HPs, the effects of CE volume (0?200 μL) and pH of LeuPNA (6 and 9) on the enzyme activities of the HP CEs of octopus, squid, and cuttlefish were studied (Fig. 3). Amidolytic activities, expressed as absorbance at 410 nm, in the same volume of Neocoleoidea HP CE toward LeuPNA (pH 6) were highest in the octopus HP CE, followed by the HP CEs from cuttlefish and squid. The amidolytic activities of all the CEs increased rapidly and linearly with increasing CE volume up to 50 μL, with slow increase thereafter. These results suggest that the optimum CE volume for measuring the amidolytic activity of exopeptidases toward LeuPNA at pH 6 is 50 μL for all the CEs. The amidolytic activities of the regions with linear increases (0?50 μL) were expressed as the following linear functions: y = 0.0068x + 0.0345 for the CE from octopus HP, y = 0.0041x - 0.0104 for the CE from cuttlefish HP, and y = 0.0035x + 0.0086 for the CE from squid HP. The amidolytic activity in all the CE volumes was highest in the CE from octopus HP, followed by the CEs from cuttlefish HP (except for 20 μL) and from squid HP.
The amidolytic activities of all the CEs from Neocoleoidea HPs toward LeuPNA (pH 9) increased rapidly and linearly with increasing extract volume up to 50 μL, with slow increase thereafter. These results suggest that the optimum CE volume for measuring the amidolytic activity of exopeptidases toward LeuPNA at pH 9.0 is 50 μL for all the CEs. The amidolytic activities of the regions with linear increases (0?50 μL) were expressed as the following linear functions: y = 0.0058x + 0.0357 for the CE from octopus HP, y = 0.0029x + 0.0428 for the CE from squid HP, and y = 0.0024x - 0.0179 for the
CE from cuttlefish HP. The amidolytic activity in all the CE volumes was highest in the CE from octopus HP, followed by the CEs from squid HP and cuttlefish HP.
Except for 20 and 50 μL of CE from squid HP, the amidolytic activities in all the samples toward LeuPNA were slightly higher at pH 6 than at pH 9, suggesting distinct proteolytic enzyme activities in most samples in the weak acid to alkali pH range.
Except for some CEs, the exopeptidase activities of most CEs from Neocoleoidea HPs were higher than their endoprotease activities. The hydrolyzing activity in 50 μL of CE from octopus HP toward LeuPNA at pH 6.0 was 0.398, which was markedly higher than the activities in the same volumes of the other samples toward LeuPNA and azocasein. Kim et al. (2007, 2008b) also found that the exopeptidase activity of viscera CE from the Argentine shortfin squid at pH 7.5 was relatively higher toward LeuPNA and ArgPNA as substrates than toward azocasein. According to reports by Heu and Ahn (1999) and Heu et al. (2003), the activities of endoproteases extracted from most fish and shrimp are stronger than those of exopeptidases.
>
Specific activities of the CEs
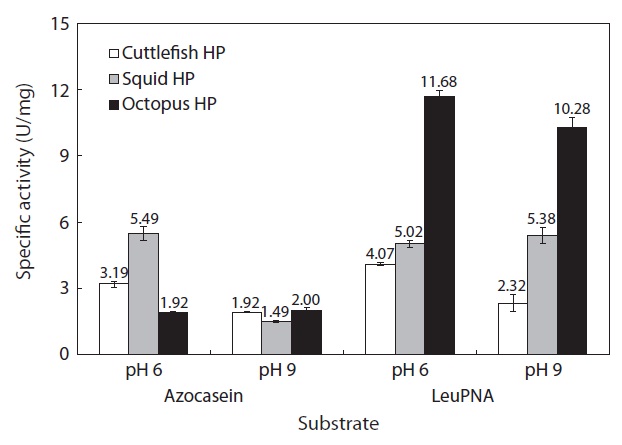
The specific activities of the CEs of octopus, squid, and cuttlefish HPs (50 μL) toward azocasein are shown in Fig. 4. Toward azocasein at pH 6 and 9, the specific activities were respectively 5.49 and 1.49 U/mg for the CE from squid HP, 3.19 and 1.92 U/mg for the CE from cuttlefish HP, and 1.92 and 2.00 U/mg for the CE from octopus HP. These results indicate that for all the CEs from Neocoleoidea HPs, the specific activity toward azocasein was higher at pH 6 than at pH 9. Heu and Ahn (1999) investigated the specific activity of the viscera CEs from 10 fish species (anchovy, bastard halibut, black sea bream, coho salmon, filefish, red sea bream, Schlegel’s black rockfish, yellow tail, mackerel, and skipjack tuna) toward casein at pH 3, 6 and 9. They reported that the specific activities of all the CEs from the fish viscera were highest at pH 8, followed by pH 6 and pH 3.
Toward LeuPNA at pH 6 and 9, the specific activities were respectively 5.02 and 5.38 U/mg for the CE from squid HP, 4.07 and 2.32 U/mg for the CE from cuttlefish HP, and 11.68 and 10.28 U/mg for the CE from octopus HP. These results indicate that the specific activities of octopus and cuttlefish HP CEs toward LeuPNA were higher at pH 6 than at pH 9. No significant differences in the specific activities of the CE from squid HP toward azocasein and LeuPNA were found.
On the basis of these results, among the Neocoleoidea HPs tested in this study, the octopus HP appears to be a superior resource for extracting exopeptidases. In general, proteolytic enzymes can be classified by optimum pH. For example, aspartic proteinases such as pepsin, gastricsin, and cathepsin D are proteolytic enzymes with strong hydrolyzing activity at acidic pH, while cysteine proteinases such as cathepsins B, H, and L are proteolytic enzymes with strong hydrolyzing
activity at weakly acidic and neutral pHs (Heu et al., 1995, 1997). Serine proteinases such as chymotrypsin, trypsin, elastase, and carboxypeptidase are also proteolytic enzymes with strong hydrolyzing activity at alkaline pH (Heu et al., 1995).
The results in this experiment and previous reports suggest that there are large amounts of cysteine proteinase, such as cathepsins B, H, and L, and cysteine proteinase-like enzymes in HP CEs from Neocoleoidea such as octopus, squids, and cuttlefish.
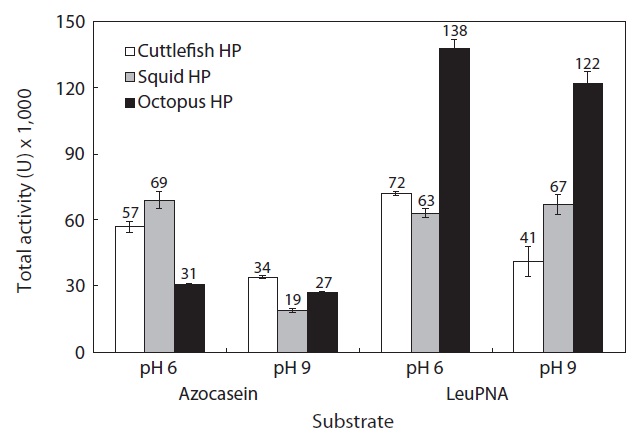
The total activities of the CEs from the octopus, squid, and cuttlefish HPs toward azocasein are shown in Fig. 5. Toward azocasein at pH 6, the total activity of the CE from octopus HP was 31,000 U, 1.84-fold and 2.2-fold lower than those of CEs from cuttlefish HP (57,000 U) and squid HP (69,000 U), respectively. The total activities of the CEs toward azocasein were higher at pH 6 than at pH 9.
Toward LeuPNA at pH 6, the total activity of the CE from octopus HP was 138,000 U, 1.92-fold and 2.2-fold higher than those of CEs from cuttlefish HP (72,000 U) and squid HP (63,000 U), respectively. Toward LeuPNA, the total activities of the CEs from octopus HP and cuttlefish HP were higher at pH 6 than at pH 9. There was no difference between pH 6 and pH 9 in total activity of the CE from squid HP toward LeuPNA.
According to the results for total activity, octopus HP is superior to cuttlefish and squid HPs as a potential resource for extracting exopeptidases. Exopeptidases from octopus HP could be used in food industry applications. Recovery from the viscera might also provide a partial solution to the pollution problem caused by the Neocoleoidea industry.