The purpose of this study was the purification and characterization of an angiotensin I converting enzyme (ACE) inhibitory peptide purified from enzymatic hydrolysates of rainbow trout Oncorhynchus mykiss muscle. After removal of lipid, the approximate composition analysis of the rainbow trout revealed 24.4%, 1.7%, and 68.3% for protein, lipid, and moisture, respectively. Among six hydrolysates, the peptic hydrolysate exhibited the highest ACE inhibitory activity. We attempted to purify ACE inhibitory peptides from peptic hydrolysate using high performance liquid chromatography on an ODS column. The IC50 value of purified ACE inhibitory peptide was 63.9 μM. The amino acid sequence of the peptide was identified as Lys-Val-Asn-Gly-Pro-Ala-Met-Ser- Pro-Asn-Ala-Asn, with a molecular weight of 1,220 Da, and the Lineweaver-Burk plots suggested that they act as a competitive inhibitor against ACE. Our study suggested that novel ACE inhibitory peptides purified from rainbow trout muscle protein may be beneficial as anti-hypertension compounds in functional foods.
Hypertension is a worldwide problem of epidemic proportions that affects 15-20% of all adults. Its treatment is one of the major risk factors for the development of cardiovascular disease, stroke, and the end stage of renal disease (Zhang et al., 2006). Among the processes associated with hypertension, angiotensin I converting enzyme (ACE) plays an important role in the regulation of blood pressure. In the rennin-angiotensin system, ACE (peptidyl dipeptidase, EC 3.4.15.1) acts on decapeptide angiotensin I (Asp-Arg-Val-Tyr-Ile-His-Pro- Phe-His-Leu) to hydrolyze His-Leu from its C-terminal and produces the potent vasopressor octapeptide angiotensin II (Asp-Arg-Val-Tyr-Ile-His-Pro-Phe). While in the kinin-kallikrein system, ACE inactivates the vasodilator bradykinin (Bougatef et al., 2010).
Many synthetic ACE inhibitors including Captopril, Enalapril, and Lisinopril among others are available for clinical use, however some undesirable side effects may occur such as cough, loss of taste, renal impairment and angioneurotic oedema (Brown and Vaughan, 1998). In the past there has been a trend toward the development of natural ACE inhibitors isolated from various organism proteins (Fujita et al., 2000; Pihlanto-Leppala et al., 2000). As a result of this research, various ACE inhibitory peptides through enzymatic hydrolysis have been isolated from marine organisms, including the skate skin (Lee et al., 2011), seaweed pipefish (Wijesekara et al., 2011), brownstripe red snapper (Khantaphant et al., 2011), tuna back bone (Lee et al., 2010), and sea cucumber (Zhao et al., 2007). Enzymatic hydrolysate showed several advantages when added to foods, such as improving water-binding ability, heat stability of myo?brillar protein, emulsifying stability, solubility of protein, and the nutritional quality of foods. Moreover, enzymatic hydrolysis has become a valuable tool for modifying the functionality of proteins (Korhonen et al., 1998). During hydrolysis, hydrophobicity of the amino-acid side chains is normally due to relatively small peptides, with molecular weights between 1,000 and 6,000 Da. Therefore, enzymatic hydrolysis was established as a source of bioactive peptides, which are short peptides released from food proteins by hydrolysis and have certain biological activities that may be beneficial for the organism (Je et al., 2005 a). Bioactive peptides usually contain 3-20 amino acid residues per molecule and are inactive within the sequence of the parent protein molecule. Moreover, bioactive peptides can be liberated by gastrointestinal digestion through proteolytic enzymes or during the fermentation process (Korhonen and Pihlanto, 2006).
The rainbow trout
The bones and viscera were removed from the rainbow trout and separated muscle was stored at -80℃ until used. Rainbow trout muscle lipid was removed using an organic solvent (n-hexane:ethanol = 1:2). ACE (from rabbit lung), hippuryl L-Histidyl-L-histidyl-L-Leucine, and various commercial enzymes, such as α-chymotrypsin, papain, pepsin and trypsin were purchased from Sigma Chemical Co. (St. Louis, MO, USA) Alcalase and Neutrase were purchased from Novo Co. (Novo Nordisk, Bagsvaerd, Denmark). All other reagents used in this study were reagent grade chemicals.
>
Analysis of approximate compositions
Crude protein content was determined by the Kjeldahl method using an Auto Kjeldahl system (B-324/435/412; Buchi, Flawil, Switzerland). Crude lipid content was determined by the ether extraction method. Moisture content was determined by oven drying at 105℃ for 24 h. Ash content was determined by a muffler furnace at 550℃ for 4 h (Association of Official Analytical Chemist, 2000). Amino acids were analyzed using an automatic analyzer (835-50; Hitachi, Tokyo, Japan) with a C18 column (5 μm, 4.6 × 250 mm; Waters, Massachusetts, MA, USA). The reaction was carried out at 38℃, with the detection wavelength at 254 nm and a flow rate of 1.0 mL/min. All chemical analyses (from each tank) were carried out in triplicate.
>
Preparation of rainbow trout muscle hydrolysate
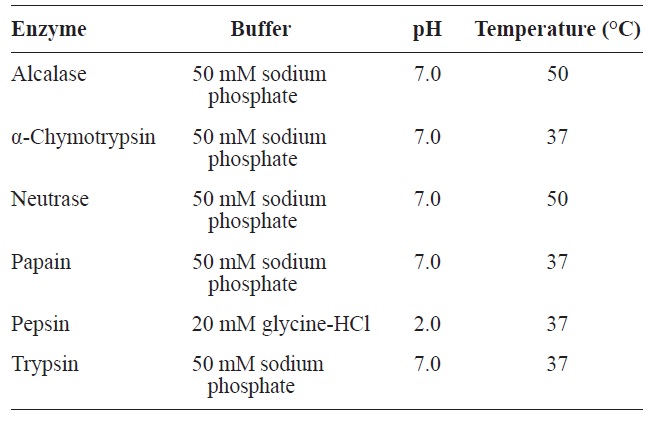
For the production of ACE inhibitory activity peptide from rainbow trout muscle protein, enzymatic hydrolysis was performed using various commercial enzymes (Alcalase, α-chymotrypsin, Neutrase, papain, pepsin, and trypsin) at an enzyme/substrate ratio of 1/100 (w/w) for 6 h, under optimum pH and temperature conditions (Table 1). After the reaction, reactant was conducted by glass filter. Degree of hydrolysis (DH) was determined by measuring the soluble nitrogen content in 10% trichloroacetic acid as followed by Kim et al. (1990), and lyophilized hydrolysates were stored at -80℃ until use.
>
Determination of ACE inhibitory activity
The ACE inhibition activity was measured using HHL as the substrate, according to Cushman and Cheung (1971) with slight modification. A 50 μL rainbow trout muscle hydrolysate solution with 50 μL of ACE solution (25 mU/mL) was pre-incubated at 37℃ for 10 min, and the mixture was subsequently incubated with 100 μL of substrate (50 mM HHL in 50 mM sodium borate buffer) for 60 min at the same temperature. The reaction was terminated with the addition of 250 μL of 1 N HCl. The resulting hippuric acid was extracted with 500 μL of ethylacetate. After centrifugation (5,000 rpm, 10 min), 200 μL of the top layer (ethyl acetate layer) was transferred into a glass test tube and dried at -80℃ for 1 h. The hippuric acid was dissolved in 500 μL of distilled water, and the absorbance
[Table 1.] Optimal conditions of enzymatic hydrolysis for various enzymes
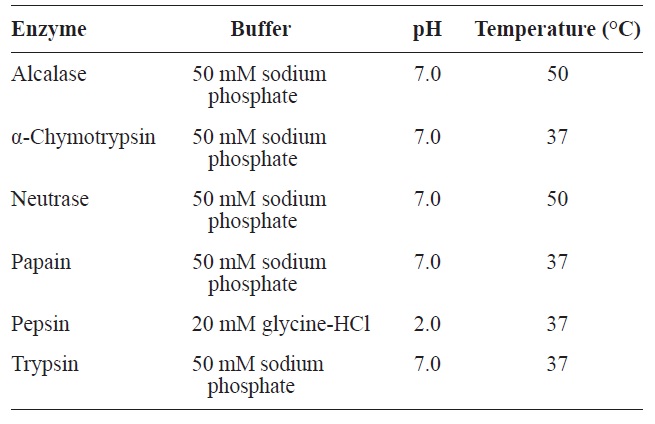
Optimal conditions of enzymatic hydrolysis for various enzymes
measured at 228 nm using a UV-spectrophotometer (V-550; Jasco, Tokyo, Japan). The ACE inhibitory activity was calculated as follows:
where Ec is the absorbance with enzyme-substrate without the sample, Es is the absorbance with enzyme-substrate and the sample, and Eb is the absorbance with enzyme and the sample without substrate. The IC50 value was defined as the concentration of inhibitor required to inhibit 50% of ACE inhibitory activity.
>
Purification of ACE inhibitory peptide
The ACE inhibitory fraction was dissolved in distilled water and separated using a preparative column (Grom-sil 120 ODS-5 ST, 5 μm, 10 × 250 mm) by reversed phase high performance liquid chromatography (RP-HPLC), with a linear gradient of acetonitrile (0-35% v/v, 30 min) containing 0.1% trifluoroacetic acid at a flow rate of 1.5 mL/min. The purified fractions from preparative column were monitored at 280 nm and purified by RP-HPLC on a C18 analytical column (5 μm 4.6 × 250 mm) using an acetonitrile gradient of 0-30% (v/v) at a flow rate of 1.5 mL/min for 40 min. Finally, the fraction with the ACE inhibitory activity was collected and lyophilized; this was followed by the identification of the amino acid sequence.
>
Identification of the purified peptide
The molecular weight and amino acid sequence of the purified peptide from rainbow trout muscle was determined using a quadrupole time-of-flight (Q-TOF) mass spectrometer (Micromass, Altrincham, UK) coupled with electrospray ionization (ESI) source. The purified peptide dissolved in methanol/ water (1:1, v/v) was infused into the ESI source and the molecular weight was determined by doubly charged (M + 2H)2+ state analysis in the mass spectrum. Following the molecular weight determination, the peptide was automatically selected for fragmentation and sequence information was obtained by tandem mass spectrometry (MS/MS) analysis.
>
Determination of ACE inhibition pattern
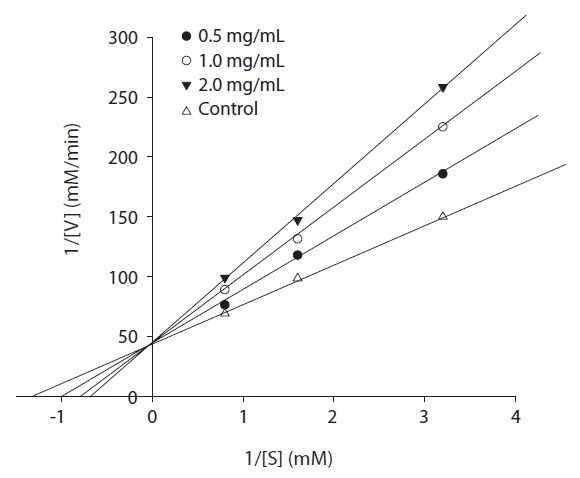
ACE inhibitor was added to each reaction mixture according to Bush et al. (1984) with some modifications. The enzyme activity was measured with different concentrations of the substrate. The kinetics of ACE in the presence of the inhibitor was determined by Lineweaver-Burk plots.
>
Proximate composition of rainbow trout muscle
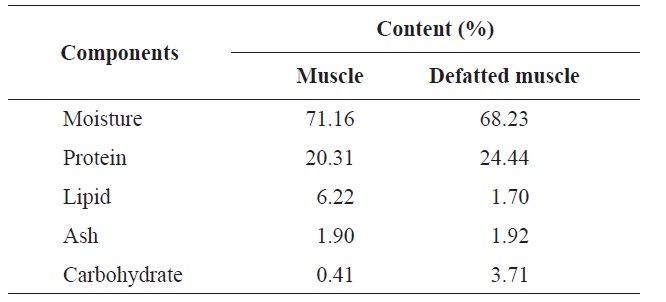
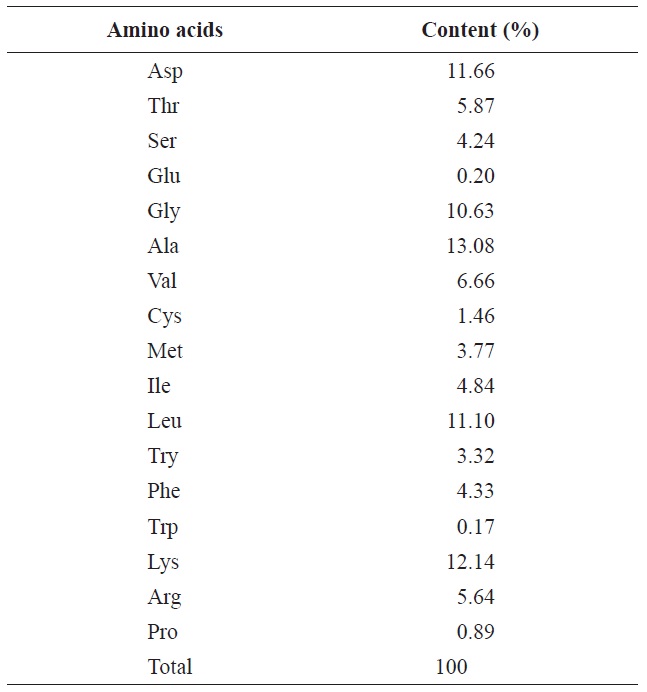
The proximate analysis of the rainbow trout muscle is shown in Table 2. The protein content was 20.31%, while the lipid and moisture contents were 6.22% and 71.16%, respectively. Gokoglu et al. (2004) reported that the approximate compositions of rainbow trout raw were 73.40% moisture, 19.85% protein, 3.41% lipid, and 1.46% ash. In comparison with our study, the protein content was similar. After removal of lipid, the protein content increased by 24.44% and through enzymatic hydrolysis was considered valuable enough to use by industry standards. The most abundant amino acids in rainbow trout muscle were glycine, lysine, aspartic acid and leucine which accounted for 10.63%, 12.14%, 11.66%, and 11.10%, respectively (Table 3). Hong et al. (2008) reported
[Table 2.] Comparison of proximate composition of muscle and defatted muscle from rainbow trout
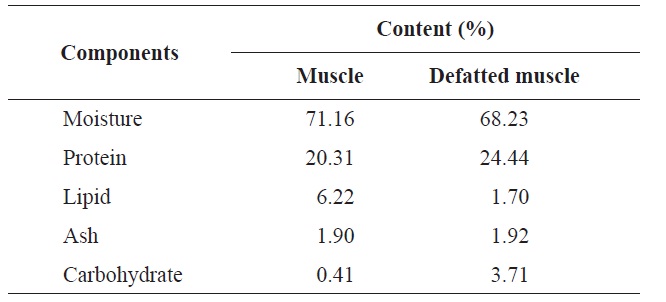
Comparison of proximate composition of muscle and defatted muscle from rainbow trout
[Table 3.] Amino acid contents of rainbow trout muscle
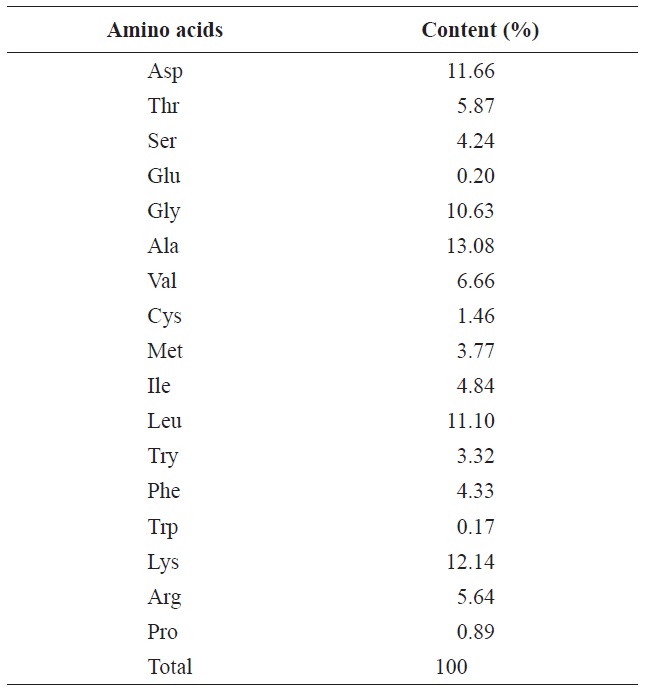
Amino acid contents of rainbow trout muscle
that many ACE inhibitory peptides contained glycine, leucine, proline, tyrosine and phenylalanine indicating that rainbow trout muscle may have ACE inhibitory peptides and exhibit potential antihypertensive activity.
>
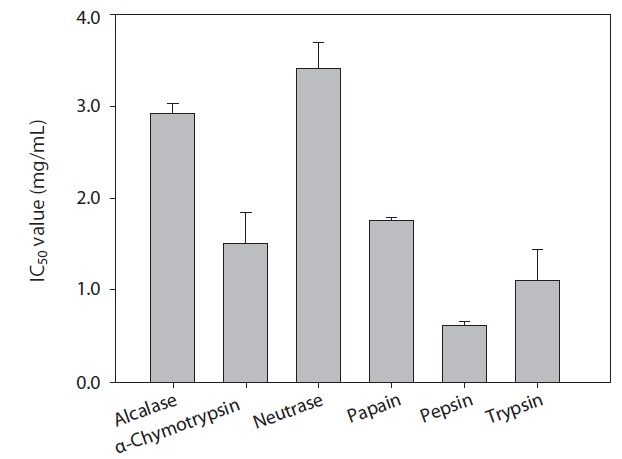
ACE inhibitory activity of hydrolysates
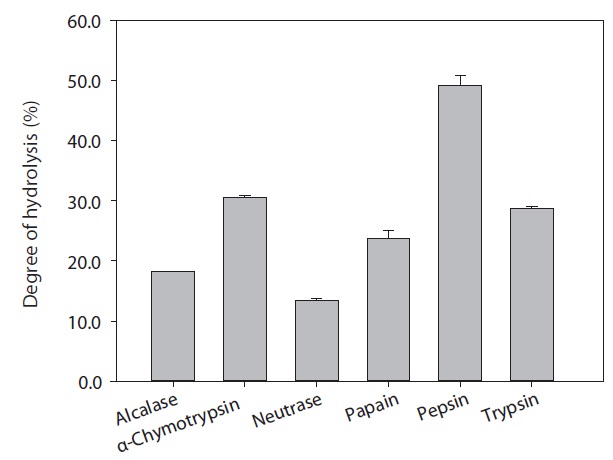
The rainbow trout muscle protein hydrolysates were prepared by hydrolysis using commercial proteases including Alcalase, α-chymotrypsin, Neutrase, papain, pepsin, and trypsin. The extent of protein degradation by enzymatic hydrolysates was estimated by evaluating the DH. Analysis revealed that DH by pepsin, α-chymotrypsin and trypsin were 49.12%, 30.52%, and 28.75%, respectively (Fig. 1). Peptides from six hydrolysates were evaluated for their ACE inhibitory activities by IC50 value (mg/mL). As shown in Fig. 2, ACE inhibitory activity of extracts produced by various enzymes, pepsin, trypsin and α-chymotrypsin were 0.61, 1.09, and 1.51 mg/mL, respectively. Among the various enzymatic hydrolysates, the peptic hydrolysate had exhibited the highestitory activity. The IC50 value was lower than reported for shrimp (3.37 mg/mL) but higher than reported for Alaska pollack protein hydrolysates (0.21 mg/mL), sardine (0.01 mg/mL) and sea bream (0.57 mg/mL) (Wijesekara and Kim, 2010). Previous report (Simpson, 2000) have shown that pepsin is one of the major fish digestive proteolytic enzymes, commonly used for industrial application. Generally, pepsin is secreted from gastric mucosa in the stomach and had preferential specificity for the aromatic amino acids, phenylalanine, tyrosine, and tryptophan. Pepsin is an endopeptidase with broad specificity produced in the mucosal lining of the stomach and degrades proteins. Pepsin is one of three principal protein-degrading, or proteolytic, enzymes in the digestive system, the other two being chymotrypsin and trypsin (Cooper et al., 1990). Recent advances in biotechnology have proved the ability of enzymes to produce novel food products, modified food composition, or improved waste processing (Lee et al., 2011). Our next step in analysis required the use of HPLC to purify theitory peptide from peptic hydrolysate of rainbow trout muscle.
>
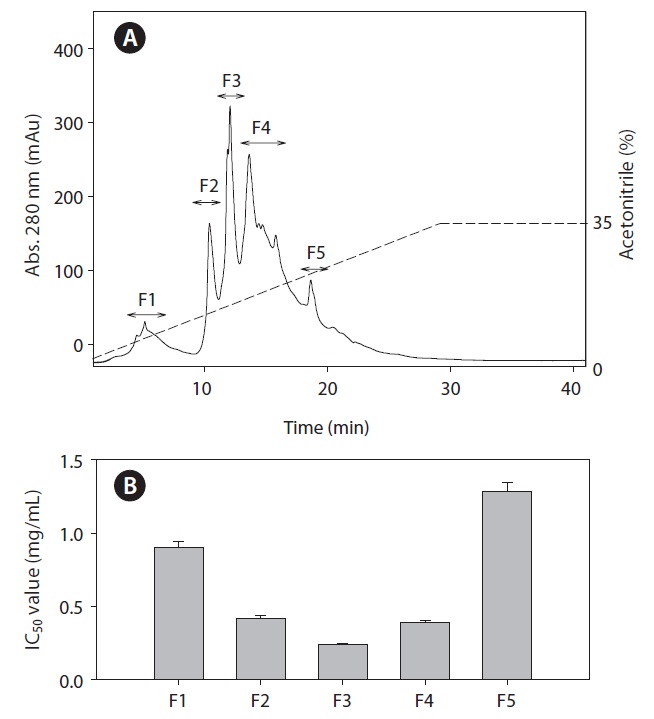
Purification of ACE inhibitory peptide
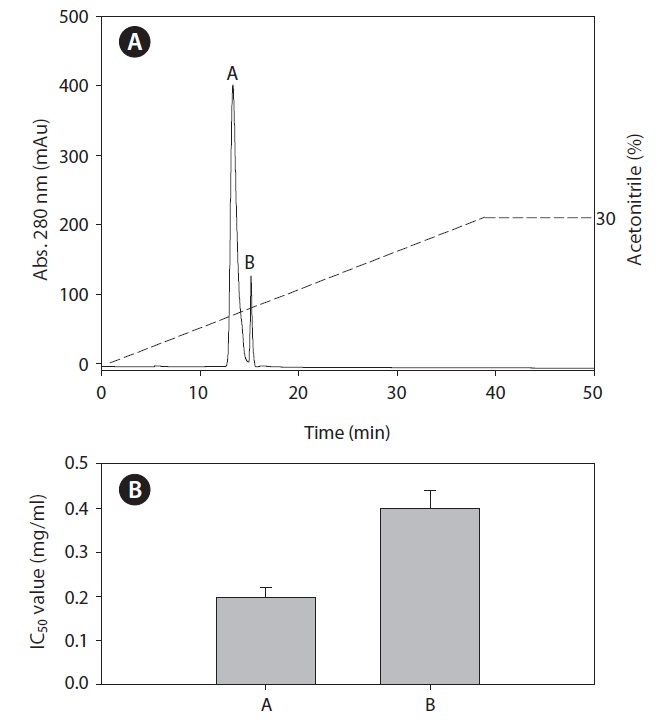
To identify the ACE inhibitory peptides derived from rainbow trout muscle hydrolysate that had the highest ACE inhibitory activity, the peptides were separated by RP-HPLC using an ODS preparative column into five fractions (F1-F5) (Fig. 3). Sub-fraction F3 possessed the highest ACE inhibitory activity. Subsequently, fraction F3 was further separated by RPHPLC using the C18 analytical column. Finally, we purified two fractions (A and B) from rainbow trout muscle hydrolysate (Fig. 4). Fraction A showed the most potent ACE inhibitory activity with an IC50 value of 0.19 mg/mL.
>
Amino acid sequence of purified ACE inhibitory peptide
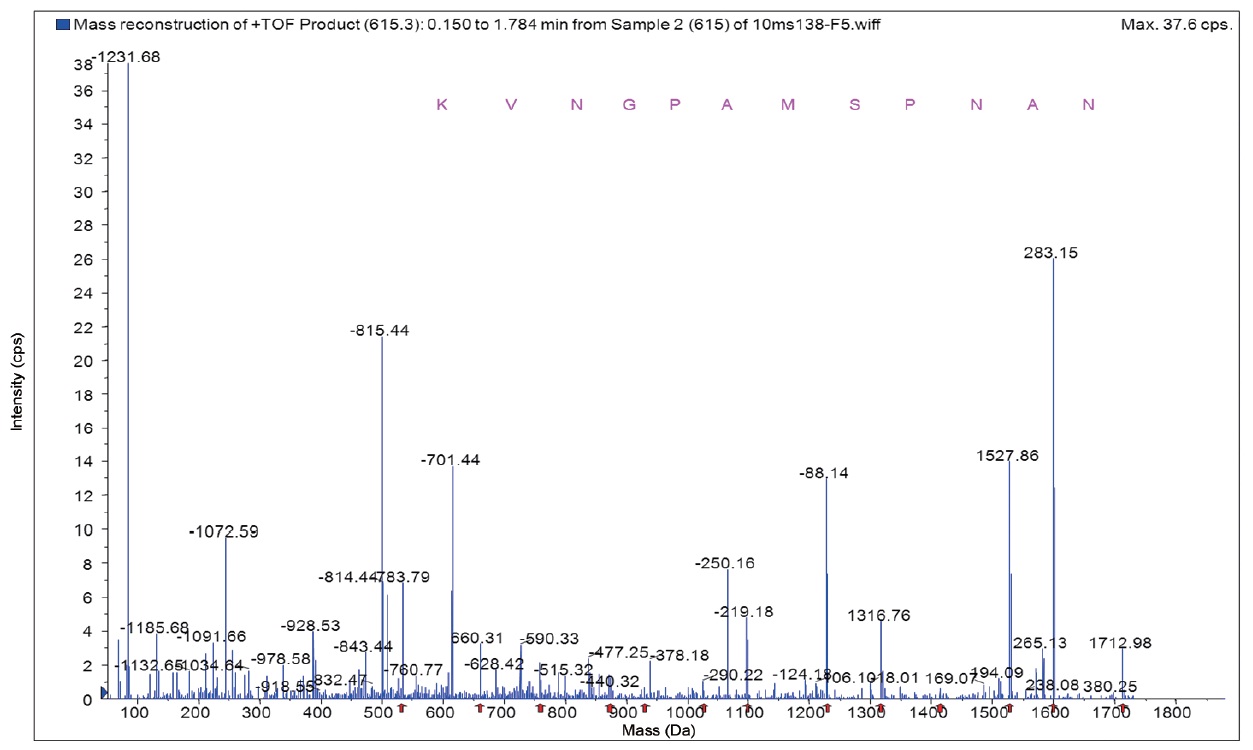
Fraction A was found to have the highest ACE inhibitory activity more than fraction B. Amino acids sequence of fraction A was identified using MS/MS and shown to be Lys-Val-Asn- Gly-Pro-Ala-Met-Ser-Pro-Asn-Ala-Asn with an ACE inhibitory IC50 value of 63.9 μM and 1,220 Da molecular weights (Fig. 5). In this study, the purified ACE inhibitory peptide was found to have a similar sequence compared to other reports, including the algae protein waste (Val-Glu-Cys-Tyr-Gly-Pro- Asn-Arg-Pro-Gln-Phe, IC50 = 29.6 μM) (Sheih et al., 2009) and sauce of fermented blue mussel (Glu-Val-Met-Ala-Gly- Asn-Leu-Try-Pro-Gly, IC50 = 2.9 μM) (Je et al., 2005b ) and porcine hemoglobin (Val-Val-Tyr-Pro-Trp, IC50 = 6.0 μM) (Yu et al., 2006). Our peptide had valine at the N-terminus which may be the reason our peptide yielded larger IC50 values. Regarding the relationship between structure and activity of ACE
inhibiinhibitory peptides, Cheung et al. (1980) reported that those peptides with valine and isoleucine at the N-terminus showed highly potent inhibitory activity. It has been confirmed that functional peptides are dependent on amino acid sequence and structure (Elias et al., 2008). Thus, the sequencing and structure of peptides could be related to ACE inhibitory activity. For example, Val-Tyr-Ala-Pro (IC50 = 6.1 μM) exhibiting a potent antihypertensive peptide was derived from cuttlefish muscle protein hydrolysate (Balti et al., 2010). Similarly, other structure-activity correlation studies have indicated that ACE binding is strongly affected by the C-terminal tripeptide sequence of the substrate and that the tripeptide could interact with subsites S1, S’1, and S’2 of ACE (Pihlanto-Leppala, 2000). The amino sequencing, strongly affects potential ACE inhibition because of the inclusion of hydrophobic amino acid residues (aromatic or branched side chains) at the C-terminal (Cheung et al., 1980). Hydrophobic amino acid residues in the ACE inhibitor sequence are a critical factor in inhibitory activity (Li et al., 2004). Therefore, we concluded that the purified peptide exhibited low ACE inhibition activity due to nondistribution of hydrophobic amino acids at the C-terminal. ACE inhibitory peptide purified from rainbow trout muscle was composed of hydrophilic amino acids at the C-terminal with an IC50 of 63.9 μM. This IC50 value exhibited lower or similar activity compared to those of peptides derived from oyster protein (Val-Val-Tyr-Pro-Trp-Thr-Gln-Arg-Phe, IC50 = 66.0 μM) (Wang et al., 2008), however, it had a higher activity than those of peptides from the hydrolysate of skate skin (Pro- Gly-Pro-Leu-Gly-Leu?Thr-Gly-Pro, IC50 = 95.0 μM) (Lee et al., 2011). The hydrophilic amino acid residues in the peptide sequence could also affect inhibitory activity by disrupting the access of the peptide to the active site of ACE. In this study, Lys-Val-Asn-Gly-Pro-Ala-Met-Ser-Pro-Asn-Ala-Asn from rainbow trout muscle contained hydrophilic amino acid such as asparagine at the C-terminal peptide sequence, which may contribute to the ACE inhibitory activity we observed.
>
ACE inhibition pattern of purified peptides
The ACE inhibition pattern of the fraction A of the purified peptides was analyzed by the Lineweaver-Burk plot and was found to be competitive (Fig. 6). Thus, ACE inhibitor from rainbow trout muscle hydrolysate binds competitively with the substrate at the active site of ACE. Moreover, the ACE inhibitory peptide from oyster was also found to be competitive (Je et al., 2005 a) along with other types such as rotifer (Lee et al., 2009). Captopril has been reported to show competitive inhibition with the substrate for binding to active ACE site (Tsai et al., 2006). These competitive inhibitors are able to enter the ACE protein molecule, interact with the active sites and prevent substrate binding (Jiang et al., 2010). Enzymes catalyze reactions in physiological systems and in equilibrium, an enzyme binds a substrate to form an enzyme-substrate complex. Enzyme inhibition is a common goal for the pharmaceutical
industry. All inhibitors cause the substrate to react at a lower rate than without the inhibitor (Lee et al., 2010). Although most reported peptide inhibitors of ACE acted as competitive inhibitors, a few have exhibited noncompetitivetype ACE inhibition (Tsai et al., 2006). The purified peptide in this study also exhibited an inhibition pattern of being able to bind to the active site. Based on the results of this study, it appears that novel ACE inhibitory peptide may be beneficial to the bioactivity of materials and functional foods related antihypertension. In this study, in order to improve the utilization of rainbow trout muscle, enzymatic hydrolysis was performed on ACE inhibitory peptides using various enzymes. The purified peptide from rainbow trout muscle was shown to exhibit potent ACE inhibitory activity with an IC50 value of 63.9 μM, and a molecular weight of 1,220 Da. The results of this study suggest that the ACE inhibitory peptide from rainbow trout muscle protein has the potential to be beneficial as a food additive or a pharmaceutical agent.