Titanium dioxide (TiO2) has been well known as an efficient phtocatalyst material [1-4]. When ultra-violet (UV) light is irradiated on, electron and hole pairs are generated in the TiO2 and they, respectively, reduce and oxidize adsorbates on the surface, generating radical species such as O2 and OH. These radicals can decompose most organic compounds [5,6], and extensive research has been performed on TiO2 in terms of applications for water and air purification [7].
In particular, the generation by UV illumination of a superhydrophilic TiO2 surface with a water contact angle (WCA) of 0° has attracted significant attention [8-13]. This material has been successfully applied as a transparent super-hydrophilic coating with anti-fogging [14] and self-cleaning properties [15]. In general, the wettability of a solid surface is well known to be governed by both the chemical composition and the geometrical microstructure of the surface [16].
In this work, the glass substrates are etched with O2 plasma prior to the coating of TiO2 in order to form the geometrical microstructure on the TiO2 surface, and the microstructure dependence of TiO2 thin films on the hydrophilic property is investigated. In particular, we focused on the effect of surface structure on the photoinduced hydrophilic properties of TiO2 films, fabricated on different surface conditions of glass substrates according to the presence or absence of O2 plasma etching. The sputtered TiO2 film has been used practically for glass with self-cleaning and anti-fogging properties.
A radio frequency (RF) magnetron sputtering apparatus was used to fabricate TiO2 film on soda-lime glass substrates with a size of 50×50 mm2. After thoroughly stirring TiO2 powder (99.99%) for 2 hours using a Ball-Mill, the powder was calcined at 500℃ in the air for 2 hours. The calcined powder was molded as a cylindrical type pellet with a diameter of 2 inch and a height of 50 mm with a pressure of 11 tons. This pellet was used as a sputter target for TiO2 films. Prior to deposition of TiO2, the glass substrates were etched with plasma for 30 min in an oxygen atmosphere of 2×10-2 Torr. The applied RF-power was fixed at 50 W for all films. The thicknesses of the TiO2 films deposited on the glass substrates were measured from 50 nm to 200 nm, which were controlled by deposition time. The surface morphologies of TiO2 films were analyzed using a field emission scanning electron microscopy (FESEM: Jeol Co.). The hydrophilicity and crystalline phase of TiO2 films were investigated by measuring the water contact angle and X-ray diffraction (XRD? Co.) pattern, respectively. The contact angle measurements were carried out at room temperature using a Kruss DSA100 goniometer following a very standard and commonly used experimental procedure as reported in the literature [17].
The photoinduced hydrophilic conversion was evaluated according to the changes in the water contact angle under UV light irradiation using black light bulbs (BLB, Toshiba lighting & Technology). The UV light irradiation was stopped when measuring the water contact angle. The photo-catalytic activity of the film was assessed for the degradation of commercial gear oil (TOYOTA gear oil super GL-5). Prior to UV irradiation, the as-deposited films were contaminated by dropping oil onto the surfaces and wiping to ensure even coverage. The samples were washed with distilled water, to remove excess oil. During the photo-catalytic degradation, the humidity was maintained at 40% RH at room temperature.
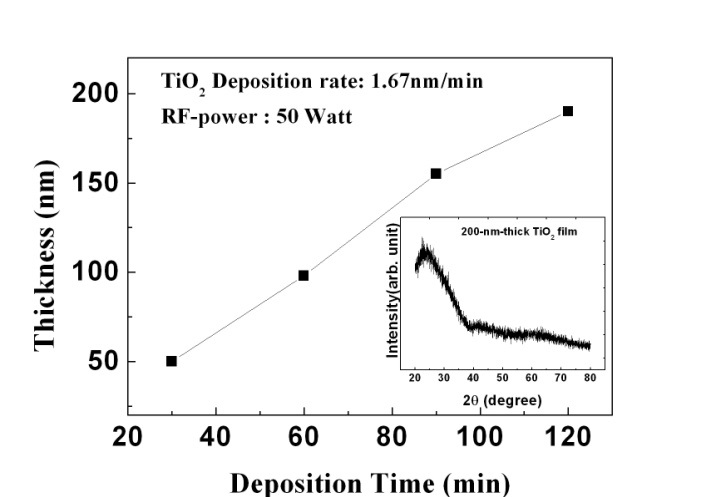
Figure 1 shows the thickness change as a function of deposition time for TiO2 films deposited on glass substrates with RFpower of 50 W. It is shown that the thicknesses of TiO2 films linearly increase as deposition time increases. Therefore, the deposition rate calculated from the slope of Fig. 1 is found to be 1.67 nm/min. In addition, the XRD pattern of the 200 nm-thick TiO2 film is represented in the inset of Fig. 1. Since no crystalline peaks are observed in the X-ray diffraction pattern, the asdeposited TiO2 films have an amorphous structure.
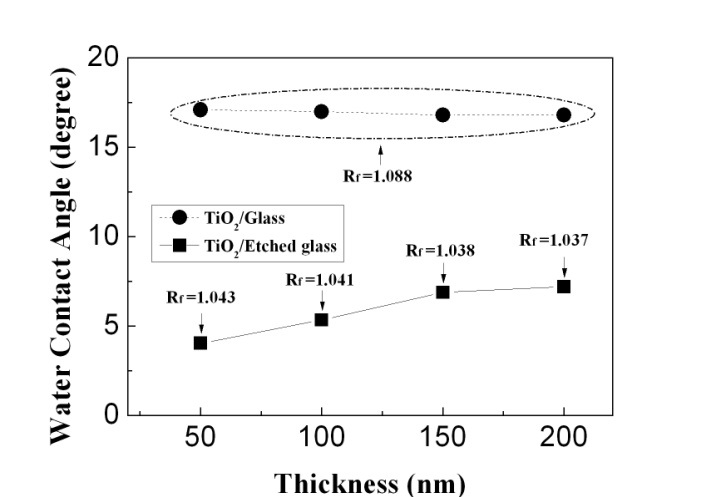
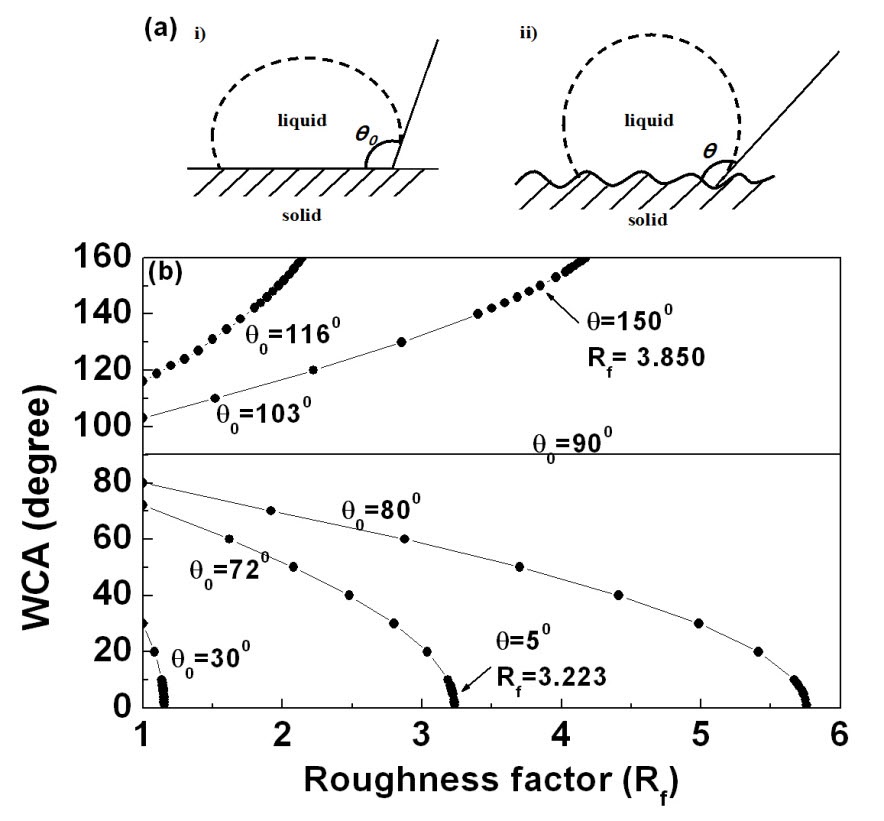
Figure 2 shows the change of WCA as a function of thickness for TiO2 films deposited on the glass etched with oxygen plasma. For comparison, the change of WCA as a function of thickness for TiO2 films deposited on the bare glass substrate is also shown. In the case of TiO2/glass, the WCAs are almost constant with a thickness of between 17° and 18°. However, TiO2 films deposited on the etched glass show lower WCAs of between 4° and 7° compared with those of TiO2/glass. This enhanced hydrophilic property of TiO2 film can be explained by Wenzel’s model. Consider a rough solid surface with a typical size of roughness detail smaller than the size of the droplet, as shown in Fig. 3(a). For a droplet in contact with a rough surface without air pockets, referred to as a homogeneous interface (or Wenzel’s model), according to Wenzel, the contact angle is given as follows.
Rf : roughness factor
θ : contact angle for the rough surface
θ0 : contact angle for a smooth surface
Figure 3(b) shows that the dependence of the contact angle on the roughness factor is predicted for various values of
(
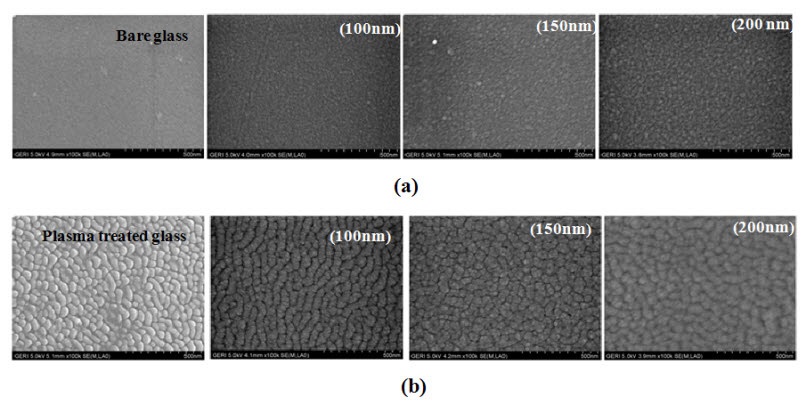
Thus, the enhanced hydrophilic property of TiO2 films deposited on the etched glass shown in Fig. 2 is attributed to the surface roughness formed after the plasma etching on the glass substrate. The roughness is observed in Fig. 4, which shows SEM images for (a) unetched glass and (b) etched glass and TiO2 films with various thicknesses deposited on them. In the case of glass substrate, nano protrusions are formed on the surface of the glass after plasma etching, as shown in Fig. 4(b), which is compared to the smooth surface of unetched glass.
A calculated roughness factor of the etched glass, based on Wenzel’s model, is 1.088, where WCAs of unetched and etched glass surfaces are considered to be 28° (=
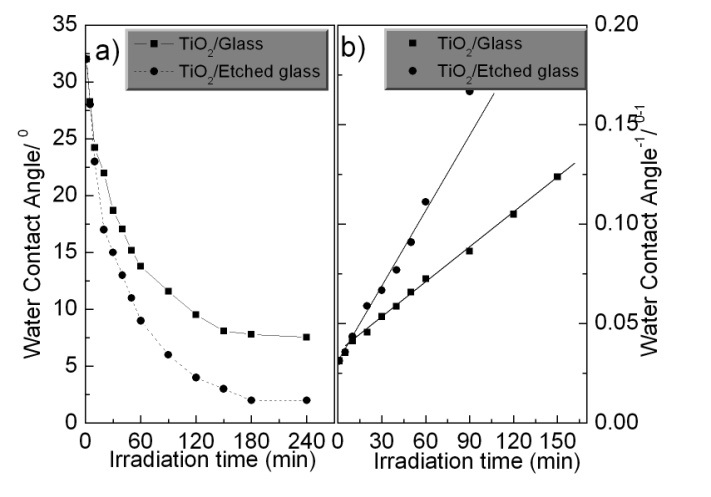
The photoinduced hydrophilic properties are shown in Fig. 5, where Fig. 5(a) indicates the changes in water contact angles on both films under UV light irradiation with intensity of 0.1 mW/ cm2. The reciprocal of water contact angles is also plotted against irradiation time, a linear relationship is obtained, as shown in figure (b). This straight line is defined as the rate constant for the hydrophilic conversion process. A distinguished difference was observed in the critical water contact angles and the rate constants for the hydrophilic conversion between TiO2/etched glass films and the TiO2/glass films. It is clear that the TiO2/etched glass film became highly hydrophilic compared to the TiO2/glass film. This indicates that the photoinduced hydrophilic reaction of the TiO2 surface was also enhanced by oxygen plasma etching on the surface of the glass.
When TiO2 is illuminated with UV light, electron and hole pairs are generated, which reduce and oxidize adsorbates on the surface, respectively. These reactions are known as photocalysis [1-6].
Thus, the enhanced photoinduced hydrophilic reaction TiO2 film deposited on the etched glass in comparison with that of TiO2 coated on the bare glass may be attributed to an increase of the light receiving area due to the nano protrusions formed on the glass after the plasma etching, which results in an increase of photogenerated electron and hall pairs.
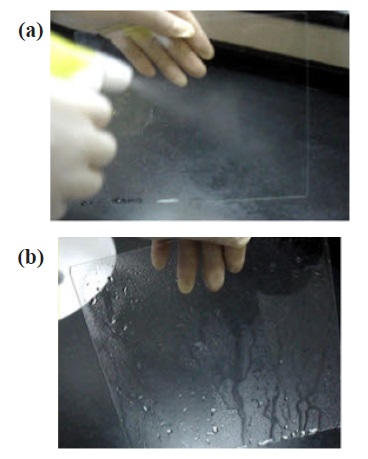
Because of the high hardness, adhesion, and highly photoinduced hydrophilic reaction of the sputtered TiO2 film, the sputtered TiO2 films are suitable for application in many outdoor uses, especially in the exterior rear view mirrors and windshields of automobiles (see Fig. 6). The TiO2 films have been used practically for large-area glass with anti-fogging [14] and self-cleaning properties [15]. These products promise aclear view on rainy days.
In this work, TiO2 films were deposited on glass substrates with and without O2 plasma etching by using the RF-magnetron sputtering method, and the wettability and sensitization of photocatalytic reactions were investigated. We focused on the effect of surface structure on the photoinduced hydrophilic properties of TiO2 films, fabricated on different surface conditions of glass according to the presence or absence of the O2 plasma etching. The surface structures of TiO2 films were observed from SEM images and their wettability and photoinduced hydrophilic properties were investigated according to the changes in water contact angles under UV light irradiations with intensity of 10 mW/cm2. As a result, the enhanced hydrophilic property of TiO2 film deposited on the etched glass can be explained by the increase of roughness factor calculated, based on Wenzel’s model.
On the other hand, the photoinduced hydrophilic properties on the TiO2 formed above the etched glass were also superior to those on the TiO2 formed above bare glass. The enhancement of
the hydrophilic conversion of the TiO2 film/etched glass can be explained by an increase of the light receiving area due to the surface structure formed on the surface of the TiO2 film, resulting in further accumulation of photogenerated electrons and hole pairs at the surface of TiO2. The sputtered TiO2 films are applicable for outdoor uses because of the high hardness, adhesion to the glass substrates, and highly photoinduced hydrophilic reaction, the TiO2 films have been used practically for glass with selfcleaning and anti-fogging properties. Therefore, we suggest that these products can be applied to the exterior rear view mirrors and windshields of automobiles to ensure clear vision on rainy days.