Composites of platinum and multiwalled carbon nanotubes (MWNTs) were prepared in various reduction conditions and characterized using cyclic voltammetry. The MWNTs were functionalized with carboxylic acid and/or hydroxyl groups in acidic solutions prior to the formation of MWNT-Pt composites. Platinum nanoparticles were deposited onto the chemically-oxidized MWNTs in 1-propanol and 1,3-propanediol. The reduction of Pt precursors in other solutions could induce differences in their morphologies in composite thin films. The morphologies of MWNTs with Pt deposited were dependent on the reduction solutions, and the electrocatalytic activities on alcohols changed accordingly. The electrochemical activities of the as-prepared MWNT-Pt thin films on common alcohols such as methanol and ethanol were investigated.
Platinum and platinum alloy materials are receiving a great deal of attention for the fuel cell technologies [1-3]. The demand for renewable energy technology such as fuel cell is expected to increase substantially due to the limitations of fossil fuels and increased needs for new and clean energies. The fuel cell is an important energy conversion system that can directly change chemical energy into electric energy without charge-discharge processes [4]. Fuel cell systems employing alcohols (Direct Alcohol Fuel Cells, DAFCs) are especially attractive as power sources for mobile applications, such as cellular phones and laptop computers, because molecularly small alcohols such as methanol and ethanol have high energy density per volume and good energy efficiency. Moreover, these small alcohols are easier to store and transfer than gaseous fuels. Some metal catalysts are feasible for electrochemically oxidizing organic compounds, and have been studied extensively for application to electrochemical power sources [5]. Platinum and platinum-based alloys have been useful metal catalysts [6,7].
Carbon nanotubes (CNTs) are important nanomaterials because of their outstanding mechanical, chemical, and electrical properties [8]. CNTs are widely studied for use as versatile building blocks for modern nanodevices, and as unique supports for various catalysts [9-14]. CNT-supported catalysts such as Ru, Pt, and Ru/Pt alloy have shown excellent activities and selectivities in various chemical reactions [15]. These have mainly been attributed to the improved catalyst stability caused by strong interaction with CNTs, which induce mass transfer or catalytic chemical states [16]. Oxidation and reduction reactions are known to favor defect sites such as five- or seven-membered rings on CNTs [17]. Pt nanoparticles well dispersed on the surfaces of CNTs have shown good activity as an electrocatalyst for methanol oxidation [18,19]. The Pt catalysts deposited on CNTs have improved the efficiency of fuel cells, and promoted the selectivity of hydrogenation [20-24]. These catalytic reactions are considerably dependent on structural factors such as the distribution and size of the nanoparticles, synthesis, and the composite morphology [25-28].
Previous results showed that some basic alcohols such as methanol, ethanol, ethylene glycol, and butanol were activated differently on multiwalled carbon nanotubes with Pt deposited (MWNT-Pt) composite thin films, depending on their structural differences [29]. In this study, MWNT-Pt composites were prepared in solutions of 1-propanol or 1,3-propanediol instead of glycerol. The reduction of Pt precursors in other solutions could induce differences in their morphologies in the composite thin films. The electrochemical activities of as-prepared MWNT-Pt thin films were deliberately investigated on common alcohols such as methanol and ethanol.
Multi-walled carbon nanotubes (MWNTs, CMP310F) with thin walls (~5-nm diameter) were supplied by Hanwha Nanotech Co. Ltd. Because of their thinner characteristics, the pristine MWNTs used in this work exist as bundles. Chloroplatinic acid hydrate (H2PtCl6·6H2O), 1-propanol, and 1,3-propanediol were purchased from Sigma-Aldrich, and ethanol, methanol, hydrogen peroxide, nitric acid, and sulfuric acid were obtained from Junsei. Water was distilled using a water purification system (Daihan Labtech Co. Ltd., LWD-108S).
600 mg of pristine MWNTs were chemically treated in 120 mL of an aqueous acid solution of 98 % H2SO4 (30 mL) and 60 % HNO3 (30 mL), in which the MWNTs were sonicated for 2 h and refluxed at 80℃ for 5 h. These acid-treated MWNTs (c-MWNTs) were washed thoroughly at least three times with distilled water to reach pH ~6, and dried in a vacuum at 60℃ overnight. In order to prepare peroxide-treated MWNTs (HO-MWNTs, which were modified with hydroxyl groups), pristine MWNTs were chemically treated in 30% H2O2 aqueous solution (sonication for 2 h and reflux at 80℃ for 5 h). Both acid- and peroxide-treated MWNTs (c(HO)-MWNTs) were prepared by the further treatment of H2O2 on c-MWNTs.
A series of MWNT-Pt composites were prepared by a method described in the literature [30]. 500 mg of H2PtCl6·6H2O was dissolved as a Pt precursor in 100 mL of 1-propanol aqueous solution (1-propanol/ H2O, 3:1 v/v), and sonicated for 2 h. 140 mg of c-MWNTs was separately added to 90 mL of 1-propanol aqueous solution (1-propanol/ H2O, 3:1 v/v) and sonicated for 1 h. Then, the Pt precursor solution was slowly added to the c-MWNT solution, and sonicated for 1 h. This solution was further refluxed for 12 h at 120-130℃ to prepare a c-MWNT composite with Pt nanoparticles (c-MWNT-Pt). The resulting solution was filtered out and dried in a vacuum oven at 70℃ for overnight. The same procedure was separately conducted to prepare the HO-MWNT and c(HO)-MWNT composites with Pt nanoparticles.
X-ray diffraction (XRD) patterns were collected by an X-ray diffractometer (PANalytical X'pert Pro MPD) equipped with reflection geometry and a fixed anode X-ray generator of a CuKα radiation source. Transmission electron microscopy (TEM) images were obtained using JEOL 2010F microscopes. TEM samples were prepared on 400-mesh carbon grids by dip-coating in dilute solutions (1 wt% solid content). Cyclic voltammetry (CV) was carried out in 0.5 M H2SO4 using an electrochemical workstation (BioLogic HCP-803) with a platinum gauze counter electrode and an saturated calomel reference electrode (SCE), and the composite thin films were deposited on a gold-deposited Si wafer. A scan rate of 50 mV/s was used for the measurement.
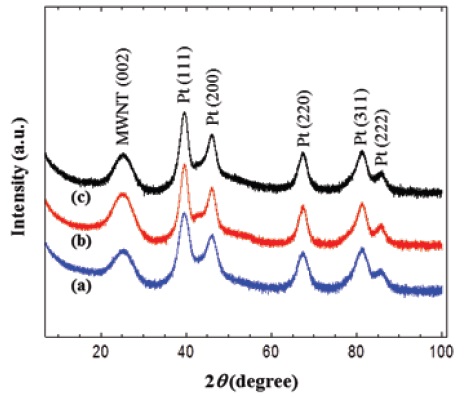
Figure 1 presents the XRD patterns of MWNT-Pt composites prepared in 1-propanol aqueous solution. Regardless of the differences in chemical modification of the MWNTs, the three MWNT composites with the Pt nanoparticles showed strong diffractions of (111), (200), (220), (311), and (222) at 39.7°, 46.4°, 67.4°, 81.4°, and 85.6°, respectively. These characteristic diffractions were similar to one another, and corresponded to Pt nanoparticles. In addition, a broad (002) diffraction peak at 25.2° was assigned to the interlayered spacing of MWNTs [31]. These XRD diffractions represent that Pt nanoparticles with good crystallinity were formed in the MWNT matrix. The TEM images of the composites also indicate good distribution of the Pt nanoparticles in the composites. The XRD patterns of the MWNT-Pt composites prepared in the 1,3-propanediol solution were also similar to those in Fig. 1. Although the reduction of Pt precursors was conducted in 1-propanol and 1,3-propanediol solutions, which have fewer hydroxyl groups than glycerol, there was no difference in the XRD patterns of the composites prepared in glycerol solution [29]. This indicates that the reduction of Pt precursors and the formation of Pt nanoparticles were also feasible for alcohols with fewer hydroxyl groups.
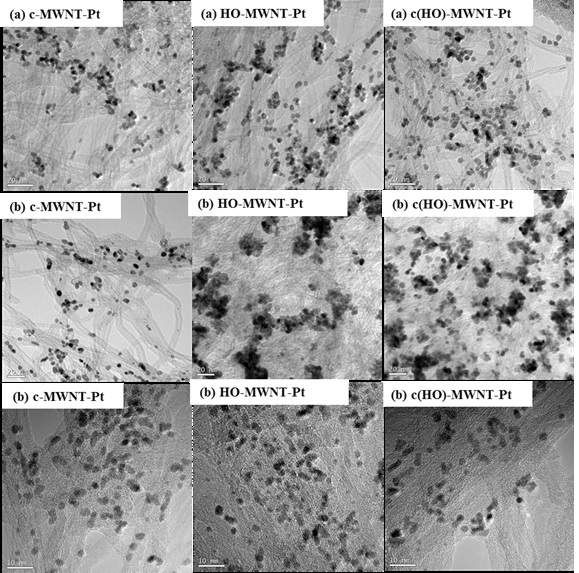
However, the distributions of Pt nanoparticles differed somewhat from one another, as shown in the TEM images of Fig. 2. Compared to the Pt nanoparticles formed in glycerol, the Pt nanoparticles formed in 1-propanol were significantly agglomerated in the MWNT matrix. The aggregation of Pt nanoparticles was especially pronounced in HO-MWNTs (Fig. 2(b)). On the other hand, the Pt nanoparticles in the c-MWNTs of Fig. 2(b) were distributed similarly to those made in glycerol (Fig. 2(a)). The aggregation and distribution of Pt nanoparticles made in 1,3-propanediol might not be dependent on the surface modification of MWNTs. The distinct difference in composite morphologies indicates that the reduction medium and chemical modification of MWNTs have an influence on the formation of Pt nanoparticles to some extent. Particularly, both 1-propanol and 1,3-propanediol have fewer hydroxyl groups than glycerol. It could be expected that the number of hydroxyl groups of the reduction solvents are related to the change in distribution and morphology of the Pt nanoparticles in the composites. Pt precursors interact with the hydroxyl groups of alcohols such as 1-propanol and 1,3-propanediol in the reaction solution to begin seeded growth, and are finally reduced to nano-scaled Pt metals. However, the reduction of Pt precursors and the formation of Pt nanoparticles were not effective in 1-propanol with one hydroxyl group, but their reduction and nano-metallization in 1,3-propanediol did not differ from that in glycerol. Glycerol has just one more hydroxyl group than 1,3-propanediol. Meanwhile, the aggregation of Pt nanoparticles made in 1-propanol was somewhat dependent on the surface modification of MWNTs. Compared to Pt nanoparticles made in c-MWNTs, Pt nanoparticles were more significantly agglomerated in HO-MWNTs. These results support that carboxylic acid groups are more effective than hydroxyl groups on the stabilization of Pt nanostructures formed on the surfaces of MWNTs.
Pristine MWNTs are simply modified with carboxylic acid and hydroxyl groups via chemical treatments, and these modified MWNTs can be further modified by various chemical treatments [32-35]. The c-MWNTs, HO-MWNTs, and c(HO)-MWNTs in this work were chemically modified by the methods described in the experimental section, and these modified MWNTs were analyzed in detail by X-ray photoelectron spectroscopy (XPS) in a previous study [29].
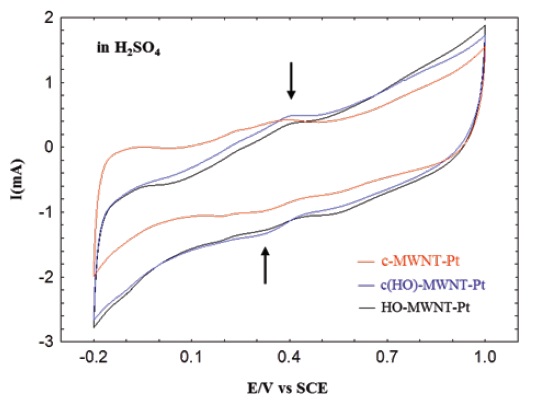
Figure 3 shows cyclic voltammograms (CV) of the MWNTPt composites measured in the absence of any alcohol. These
composites were prepared in 1-propanol solution. Although no specific signal of electrocatalytic oxidation/reduction was found in the background CV curves, the capacitive currents were relatively high due to the high active surface areas of the carbon nanotubes themselves. It was not clear, but weak peaks originating from oxidation and reduction were found from 0.3 to 0.4 V, which were caused by the electrochemical redox of the oxidized groups (-OH and -COOH) functionalized on the surfaces of MWNTs [36]. MWNT-Pt composites prepared in 1,3-propanediol also presented similar results, as shown in Fig. 3.
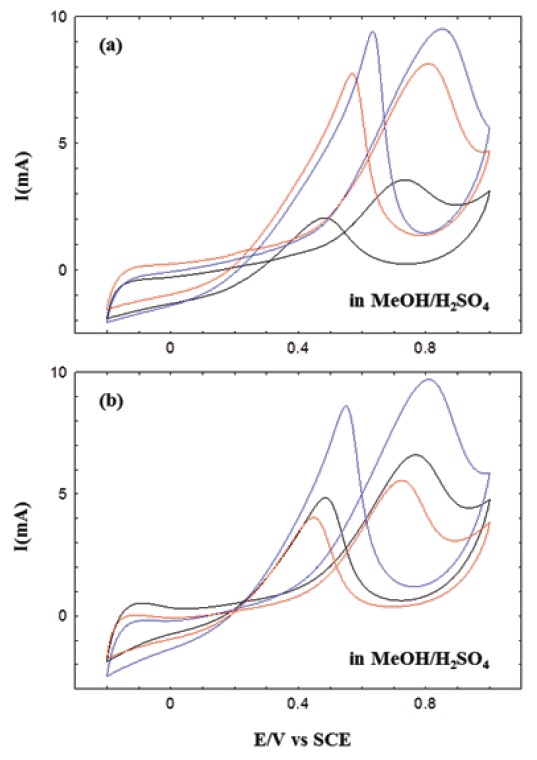
The electrocatalytic oxidation behaviors measured in alcohols have important implications for fuel cell applications. Therefore, cyclic voltamograms of methanol and ethanol were analyzed in H2SO4 aqueous solution to investigate the electrocatalytic activities of MWNT-Pt composite films. Figure 4 shows the CV results of methanol in the composite films. The CV was conducted in H2SO4 aqueous solution with methanol. Compared to Fig. 3, the cyclic currents were highly improved, and distinct oxidation peaks were identified in the CV curves of Fig. 4. A higher potential in forward scanning corresponds to the oxidation of
alcohol and the generation of CO2, where as a lower potential in backward scanning corresponds to water adsorption and activation on the Pt surfaces. A release of CO2 during alcohol activation was caused by reaction of the activated water and the oxidized form of alcohol on the Pt surfaces [37,38]. The entire CV curves showed similar shapes, regardless of the reduction solution, but their currents and peak positions were changed with the chemical modification of MWNTs. In the CV measurement of methanol, for example, the oxidation moved towards a higher voltage, and peak current gradually increased with the variation of chemically- modified MWNTs in the composite films (Figs. 4(a) and 4(b)).
In addition, c(HO)-MWNT-Pt showed the highest oxidation currents and voltages, regardless of the reduction solution. These results indicate that the catalytic activation of MWNT-Pt composites in methanol have been influenced by the surface conditions of chemically-treated MWNTs and the morphologies of Pt- MWNT composites. We have obtained similar results in previous studies on MWNT-Pt composites prepared in glycerol solution [29].
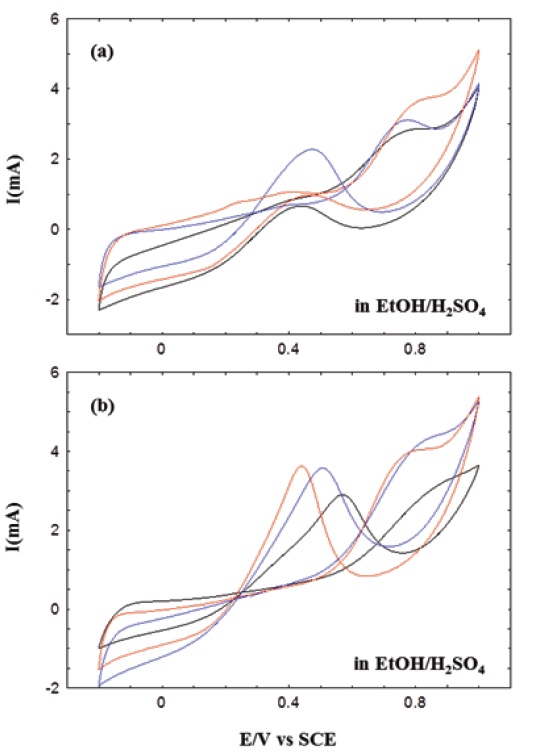
Characteristic cyclic voltammograms were separately collected in ethanol, as shown in Fig. 5, and their currents and peak positions were also changed with the chemical modification of MWNTs. The oxidation peaks of these CV curves are comparable with those previously obtained [39]. Similar to the results of methanol above, we could find two distinct oxidation peaks in ethanol solutions. However, these oxidation peaks were relatively weak, resulting from the lowered catalytic activities. The catalytic activities of
MWNT composites with Pt nanoparticles were prepared and characterized structurally and electrochemically. MWNT-Pt composites in this work were prepared in 1-propanol and 1,3-propanediol solutions instead of glycerol. Prior to the composite preparation, pristine MWNTs were chemically treated with acidic and/or hydrogen peroxide solution to achieve various modifications of the surfaces of MWNTs. Morphological change of the Pt nanoparticles was evident on the MWNT-Pt composites made in 1-propanol. Cyclic voltammograms showed that methanol and ethanol were electrochemically activated with the composites. The electrocatalytic activities of the composite systems were relatively effective in methanol, but not clear on the morphological effect of Pt nanoparticles in the composites. The electrocatalytic activities of c(HO)-MWNT-Pt improved in methanol and ethanol. The hydroxyl groups of alcohols and the oxidized groups on MWNTs were related to the catalytic activity of MWNT-Pt composites.