Pb(Zr,Ti)O3 (PZT) ceramics have been widely used for piezoelectric applications, such as filter, resonator, actuator, and sensors owing to its excellent piezoelectric properties for several decades [1,2]. However, the use of Pb material in piezoelectric devices is inhibited by environmental pollution in recent years. Therefore, alternatives for Pb-based piezoelectric ceramics are extensively requested and studied. Among them, alkali niobatebased materials such potassium sodium niobate ((Na, K)NbO3, hereafter NKN) is a promising material due to their excellent piezoelectric properties and high Curie temperature (Tc) [3]. However, NKN ceramics is difficult to obtain have difficulty obtaining high density ceramics due to volatilization of Na and K when sintered above 1,000℃. Thus, control of volatilization of Na and K or counterbalance of decrease regarding piezoelectric properties is needed in NKN systems. Thanks to the efforts for the wide research of new piezoelectric ceramic materials for several years, much information was built up in fields of new piezoelectric ceramic materials and processes [4,5]. BaTiO3 (BT) ceramic is a good lead-free piezoelectric ceramic having high piezoelectric properties. However, BT has handicaps in the views of low Tc (about 120℃) and high sintering temperature (above 1,300℃). Nevertheless, BT will be a good material to compensate for the weakness of NKN ceramics. Therefore, NKN-BT ceramics compositions were widely studied to achieve high performance Pb-free composition by varying compositions and fabrication condition [6-8]. From a report about the pure NKN-BT system, 0.95NKN-0.5BT composition has superior characteristics with excellent piezoelectric properties, high Tc and so on. Nevertheless, there is a lack of information about phase transition characteristics when sintered at high temperature and capacitance change at below room temperature for applications in using resonance characteristics in cold areas. TCC is a very important property for piezoelectric devices because the resonance frequency of the piezoelectric device is changed by capacitance.
Therefore, the purpose of this article is to investigate phase transition characteristics of NKN-BT based ceramics sintered at high temperature. At the same time, we investigated TCC of NKN-BT based ceramics sintered at high temperature in range from -40℃ to 100℃.
The starting materials used for the synthesis of (Na,K)NbO3 ceramics were as follow : K2CO3 (Junsei Chemical, Japan, 99.0%), Na2CO3 (Kanto Chemical, Japan, 99.5%), Nb2O5 (Junsei Chemical, Japan, 99.9%), BaCO3 (Kanto Chemical, Japan, 99.0%), TiO2 (Hanawa Chemical, Japan, 99.0%), Ag2O (Kanto Chemical, Japan, 99.0%). The intended compositions are 2 types: the one is 0.95(Na0.5K0.5) NbO3-0.05 BaTiO3 + 0.2 wt% Ag2O (No excess NKN) and other 0.95(Na0.5K0.5) NbO3-0.05BaTiO3 + 0.2 wt% Ag2O with 0.03 (Na0.5K0.5) NbO3 (Excess NKN). All of the starting materials were weighed according to the intended compositions and The intended compositions are 2 types: the one powder was dried in an oven at 100℃ for 24 hours. In order to enhance the uniformity on the compositions, the mixtures were calcined at 850℃ for 3 hours. The calcined powders were mixed with 3 wt% polyvinyl alcohol (PVA) solution and then pressed into pellets with a diameter of 12 mm under 1.5 ton pressure. The disks were sintered at 1,100℃, 1,150℃ and 1,200℃ for 2 hr with a rising rate of 3℃/min at atmospheric pressure. In addition, silver pastes were fired on both surfaces of sintered specimens at 600℃ for 30 min as the electrodes.
The bulk density of the sintered specimens were measured and calculated by the Archimedes method. The crystal structures of all sintered specimens were examined by X-ray diffraction (XRD, Smart Lab, Rigaku, Japan) with Cu Kα radiation (step:0.01o) to analyze the phases of sintered specimens. To perform accurate peak-fitting analysis, we used PDXL software at XRD analysis. A JSM-7001F scanning electron microscope (SEM) was used for the microstructural analysis of the specimens. The dielectric properties, capacitance and TCC of unpoled specimens were obtained using an Impedance Analyzer (HP 4294A) and temperature oven by measuring the capacitance from -40℃ to 100℃, 1kHz.
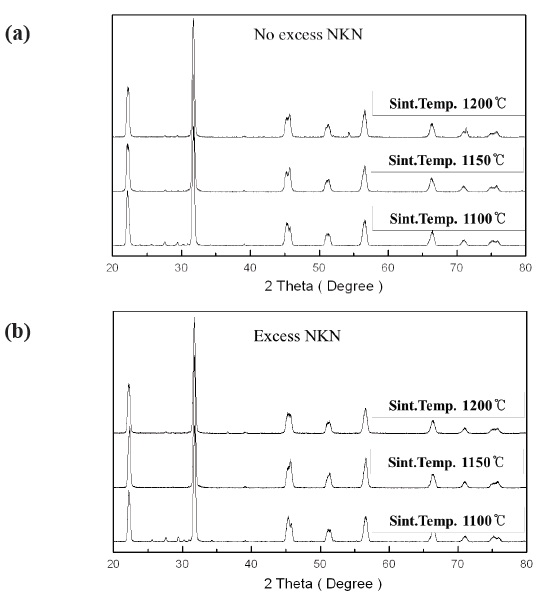
Figure 1(a) and 1(b) show XRD patterns of 0.95(Na0.5K0.5)NbO3-
0.05BaTiO3 + 0.2 wt% Ag2O (No excess NKN) and 0.95(Na0.5K0.5) NbO3-0.05BaTiO3 + 0.2 wt% Ag2O with excess 0.03 (Na0.5K0.5)NbO3 (Excess NKN) ceramics sintered at 1,100℃, 1,150℃ and 1,200℃ for 2 hr. All the compositions with variations of sintering temperature showed a single-phase perovskite structure, although there were some secondary phase peaks. This suggests that Ba, Ti and Ag ions entered into the NKN lattice within the sintering temperature ranges, with Ba2+ and Ag+ occupying A-sites and Ti4+ entering B-sites. Especially, we have to take note of the change in crystal structure by increase of sintering temperature on both compositions. We can see that the increase of sintering temperature brings about change of the crystal structure on both conditions. No excess NKN and Excess NKN sintered at 1,100℃ have an orthorhombic crystal structure at room temperature. However, with an increasing sintering temperature above 1,100℃, all the compositions show a phase transition from the orthorhombic to tetragonal phase.
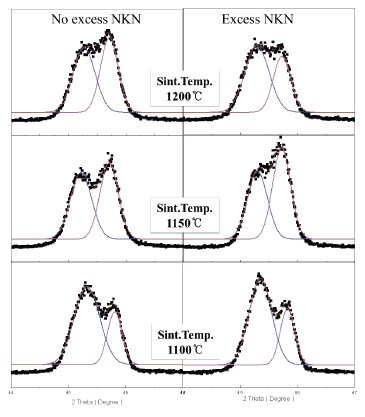
We can more clearly see the change of crystal structure from the variation of (002) and (200) peaks in Fig. 2. Also, we can expect the decrease of grain size by observing the broadening in the width of peaks with increasing sintering temperature.
Therefore, it turned out that No excess NKN and Excess NKN ceramics in this study show a PPT characteristic at a range of sintering temperature from 1,100℃ to 1,150℃.
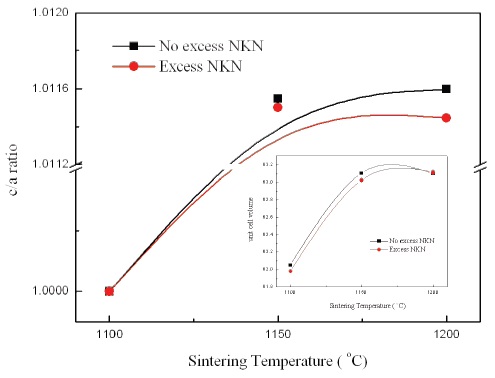
Figure 3 presents a variation of the c/a ratio with increasing sintering temperature. As shown in Fig. 3, the c/a ratio was increased and then became gradually saturated with variation of sintering temperature in No excess NKN and Excess NKN. It can be explained by a PPT characteristic of NKN ceramics and excess NKN ceramics fabricated in this study. The insert in Fig. 3 shows the change of unit cell volume with variation of sintering temperatures. Especially, the specimen sintered at 1,200℃ in Excess NKN showed slight expansion of unit cell volume in virtue of increase of the a-axis lattice constant owing to occupation of the A-site by excess Na+ and K+ in Excess NKN.
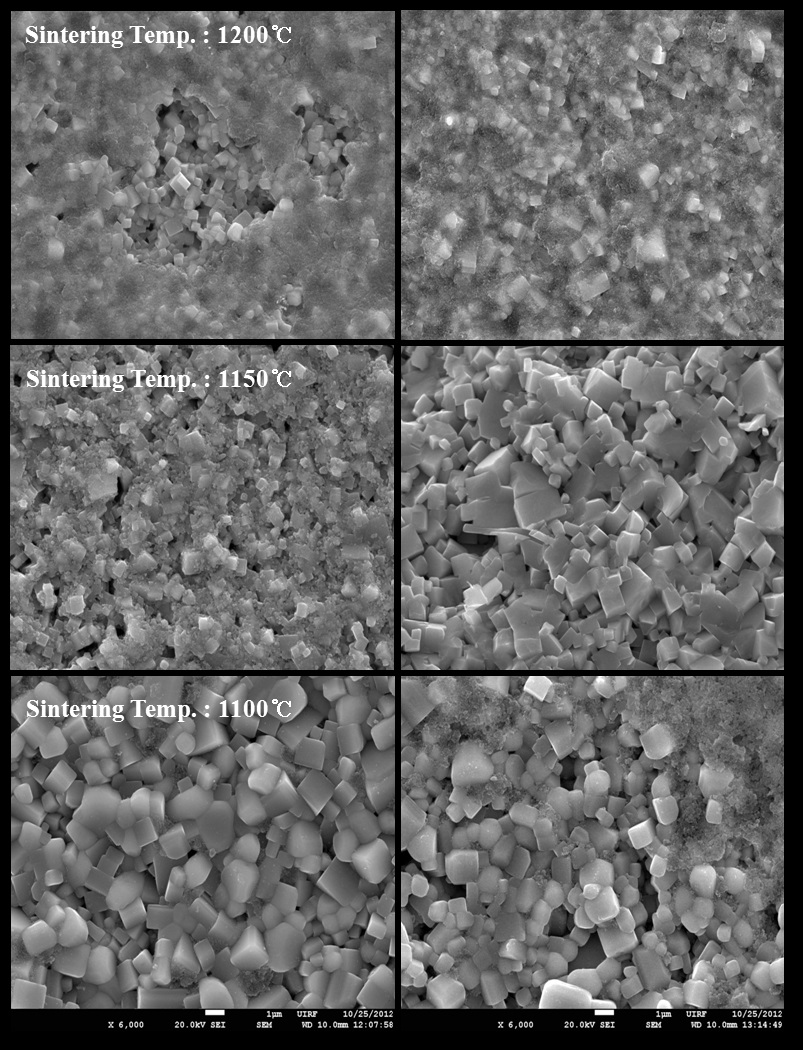
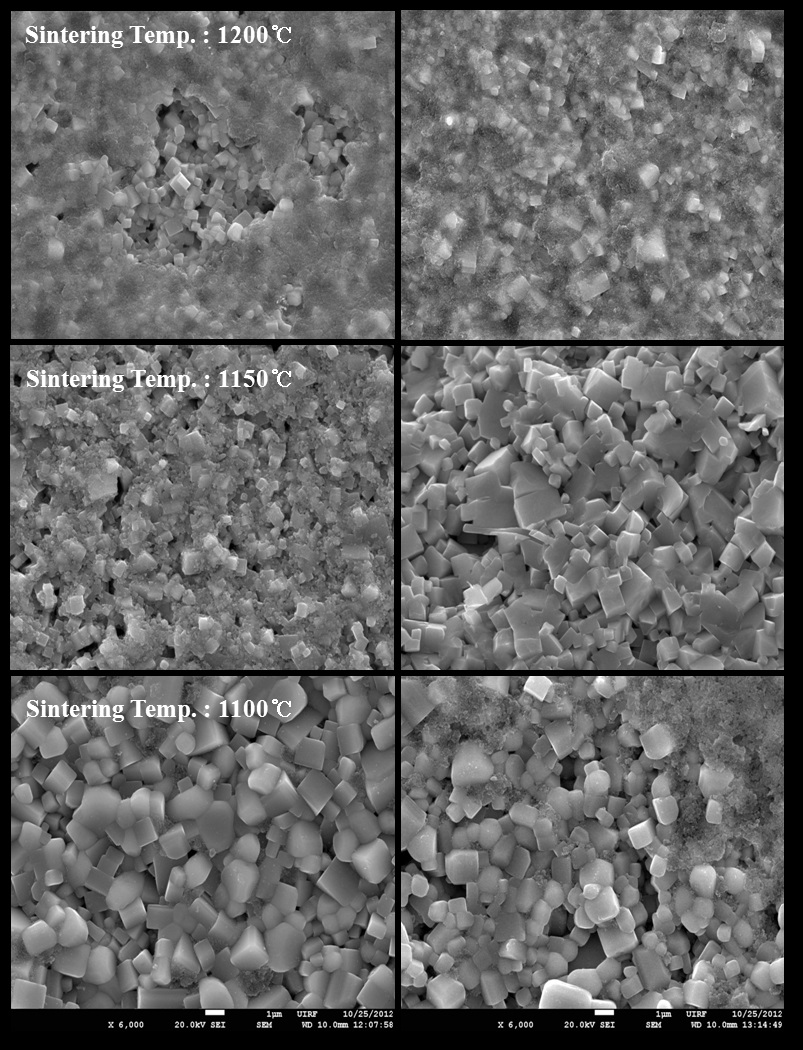
The SEM images on the fractured surface by variation of sintering temperature are shown in Fig. 4. Liquid phase formation was observed in almost all specimens. It is well known that sintering temperature in NKN based ceramics not only influences the crystal structure but also changes the microstructure of ceramics. Also, it was reported that the liquid phase, particularly
formed due to volatilization of alkaline materials, assists the densification of specimens [8].
As shown in Fig. 4, the grain shapes had a faceted type and average grain sizes were abruptly decreased with increasing sintering temperature in both compositions. This is well consistent with expectation in the XRD result. The grain sizes of two compositions had 2~2.5 μm in the specimens at sintered 1,100℃, and large pores were observed as increasing sintering temperature in No excess NKN. It might be contributed by the formation of the liquid phase due to sintering temperatures over 1,150℃. On the other hand, it became more dense and small, because the liquid phase played key roles of inhibitor for grain growth as increasing sintering temperatures in Excess NKN [9].
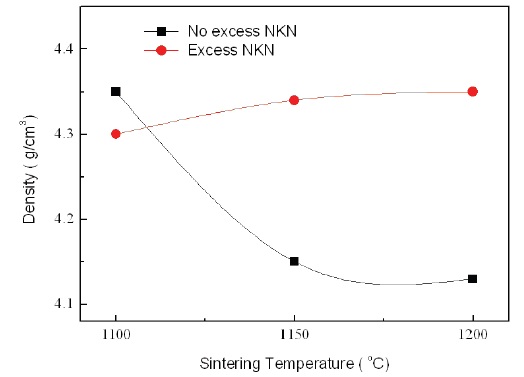
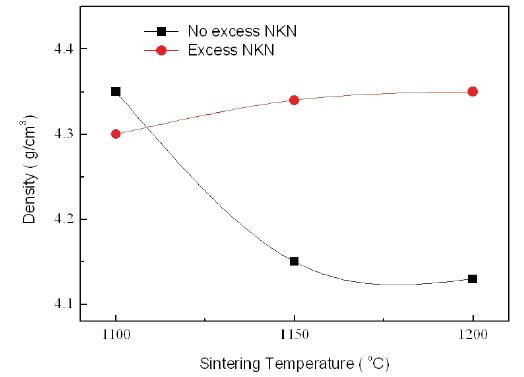
Figure 5 shows the relative density of No excess NKN and Excess NKN with increasing sintering temperatures. With increasing sintering temperatures, the relative density in No excess NKN was abruptly decreased and then gradually saturated. It is related with volatilization of alkali materials and formation of pores. However, the relative density in Excess NKN was slightly increased. The results can be explained by the densification of the microstructure as shown in Fig. 4. This tendency is well agreed with analysis of SEM results.
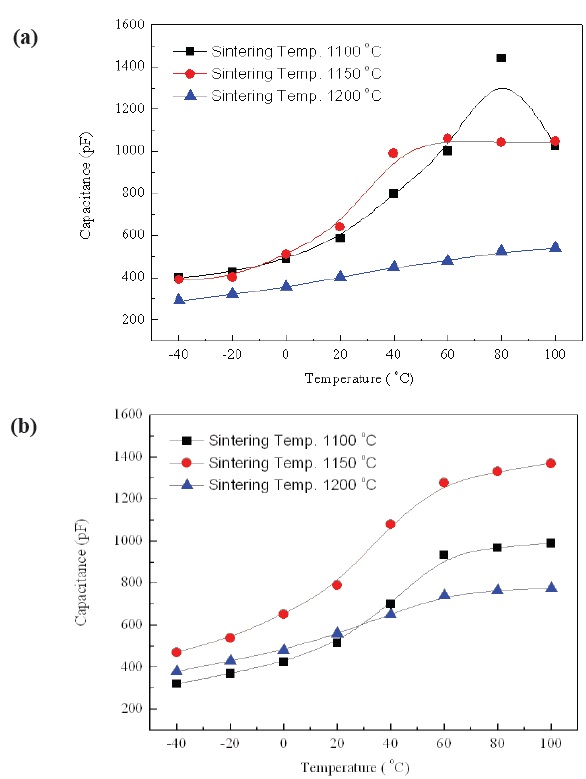
The temperature dependence of capacitance with increasing sintering temperature in No excess NKN and Excess NKN are shown in Fig. 6(a) and (b). As we can see, the capacitance of specimens with Excess NKN showed a higher value than that of No excess NKN, except specimens sintered at 1,100℃. It can be explained by an increase of relative density in Excess NKN due to the densification by liquid phases. This result is well consistent
[Fig. 4.] SEM images of No excess NKN(a) and Excess NKN(b) with variation of sintering temperatures.
[Fig. 5.] Relative density of No excess NKN and excess NKN with variation of sintering temperatures.
with the relative density shown in Fig. 5.
It is well known that the Curie temperature (hereafter, Tc) of pure NKN ceramics is around 420℃, and phase transition from the orthorhombic to tetragonal phase (hereafter, T
Also, it was reported that Tc and T
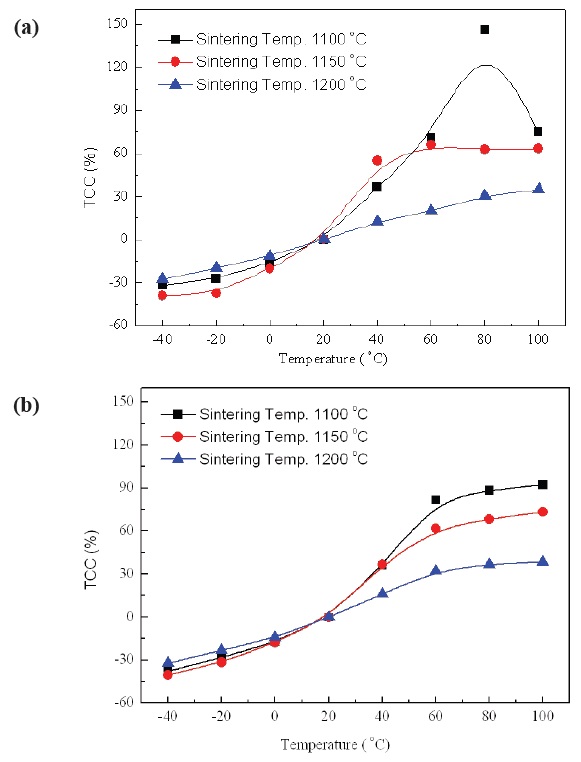
Figure 7(a) and (b) show the TCC characteristics of No excess NKN and Excess NKN in the temperature range from -40℃ to 100℃ with variation of sintering temperature.
TCC is key factor for practical applications of piezoelectric devices owing to stability of resonance frequency. A resonance frequency can be fluctuated by only a few small variations of capacitance. As shown in Fig. 7(a) and (b), TCC was changed from -40% to 150% in No excess NKN, while that of Excess NKN was
varied from -40% to 90% in a temperature range from -40℃ to 100℃. The improvement of TCC characteristic in Excess NKN, compared with No excess NKN, can be interpretted by increase of relative density in Excess NKN as shown in Fig. 5.
Therefore, to compensate for the positive TCC characteristics of NKN ceramics, it is recommended to design NKN ceramics with temperature compensated capacitor having a Class I type ceramic capacitor, high capacitance, negative temperature dependence and high tolerance of capacitance, when considered for usage of NKN based piezoelectric ceramics in cold regions.
We investigated the polymorphic phase transition and temperature coefficient of capacitance of 0.95(Na0.5K0.5)NbO3- 0.05BaTiO3 + 0.2 wt% Ag2O and 0.95(Na0.5K0.5)NbO3-0.05BaTiO3 + 0.2 wt% Ag2O with excess 0.03(Na0.5K0.5) NbO3 ceramics sintered at 1,100℃, 1,150℃ and 1,200℃ for 2 hr.
Both systems showed a change of crystal structure from the orthorhombic to tetragonal phase at a range of sintering temperature from 1,100℃ to 1,150℃, indicating a polymorphic phase transition characteristics. Also, the temperature coefficient of capacitance characteristics of both systems demonstrated positive properties from -40℃ to 100℃. Therefore, co-use of the temperature-compensated-capacitor (Negative characteristics) is recommended for the usage of piezoelectric devices in cold regions