The Water Framework Directive (WFD: European Union 2000) has precipitated an extensive body of research across the European Union (EU) as Member States attempt to develop monitoring tools capable of assessing ecological status in all freshwater and marine habitats (Noges et al. 2009, Birk et al. 2012).
The outcome is a suite of methods that can be used by a Member State to classify water bodies into one of five ecological status classes: high, good, moderate, poor or bad. Those water bodies that are classified as moderate status or lower then require that State to implement a “Programme of Measures” (PoMs) in order to restore those water bodies to at least good status, and also to ensure that water bodies do not deteriorate in status over time. One of the “biological quality elements” specified in the WFD for assessment of rivers and lakes is “macrophytes and phytobenthos”. As diatoms are a major component of phytobenthos, and as a number of methods for using diatoms to assess water quality had already been developed, many states used diatoms in order to meet these obligations.
Prior to the WFD, the UK had used the Trophic Diatom Index (TDI: Kelly & Whitton 1995) as part of a suite of techniques for determining whether phosphorus-stripping was required for large sewage works discharging to nutrient sensitive areas, as designated by the Urban Wastewater Treatment Directive (UWWTD: European Community 1991). The TDI was also used for some investigations related to the Habitats Directive (European Community 1992) and other purposes such as investigating localised sources of pollution. Consistency in sampling and analysis was ensured by adherence to international standards (CEN 2003, 2004).
Experience from these activities led to a revised version (Kelly et al. 2001) and this, in turn, was the foundation on which a new tool for WFD monitoring, known as DARLEQ (Diatoms for Assessing River and Lake Ecological Quality), was based . This uses a recalibrated version of the TDI (Kelly et al. 2008a) for rivers along with a companion metric, the Lake TDI (LTDI) for use in standing waters (Bennion et al. 2012). However, in order to make the system effective for the WFD, new work was also required to define “reference conditions” (Yallop et al. 2009, Pardo et al. 2012, Kelly et al. 2012), to relate the metric to the concept of ecological status (Kelly et al. 2008b, 2009c), to understand the uncertainty associated with the metric (Kelly et al. 2009b) and to intercalibrate it with metrics developed in other countries (Kelly et al. 2009a). More recently, a metric to assess acidification has been added (Juggins & Kelly 2012) and the taxon list has been simplified, in order to make the task of analysis more straightforward (Kelly & Yallop 2012).
As the development of the TDI and DARLEQ has already been described in a number of papers, this paper focusses on how the method was integrated into the working practices of the agencies responsible for implementing the WFD in the UK, answering to four separate administrations (England, Wales, Scotland and Northern Ireland), with central co-ordination provided by UK Technical Advisory Group (UK TAG: www.wfduk.org). One of these administrations shares a border with the Republic of Ireland, necessitating cross-border co-operation to ensure consistent application of the WFD in catchments that straddle the border.
Ecologists in these agencies form regional teams each with extensive knowledge and a long collective “memory” of a small geographical area. The core methods until the 1990s were based on family-level identification of invertebrates, developments of which culminated in River InVertebrate Prediction And Classification Scheme (RIVPACS: Wright et al. 1989) which provided a powerful means of classifying rivers and for informing water management on issues associated with, in particular, organic pollution.
Throughout the 1990s, teams embraced a range of other techniques: macrophyte or hydromorphological survey, phycology and diatom analysis, and fish survey work. A typical team-member now might be trained in two or three of these skills. Not all work is done in-house, with contractors used for some more specialised work and routine analyses, when local capacity is exceeded. However the combination of local knowledge and broad technical expertise provides a valuable foundation for the biologists to advise and support catchment management.
Although the UK agencies had used diatoms for assessments related to the UWWTD since 1995, there was little in-house analytical capacity. Local staff collected samples which were mostly sent to contractors for analysis. Interpretation was mostly limited to calculation of the TDI. The long-term objective of implementation of DARLEQ was to develop in-house capacity in all aspects, from sampling through to data interpretation in support of the decision-making process.
Basic training in diatom sampling, slide preparation, species identification and interpretation was provided by a distance-learning course Introduction to Freshwater Diatoms, developed and managed by Bowburn Consultancy in the 1990s. This consisted of six modules which took students through a series of tutorials guiding them in the essentials of diatom biology and identification whilst, at the same time, teaching them basic techniques. The course included a box of 12 permanent slides, each of a sample with a range of common taxa. The tutorial notes are based around a simple binomial key (Kelly 2000) and also an interactive CD-ROM-based key (Environment Agency 2007). Students perform a series of exercises on samples collected from local streams, leading up to analyses of slides and a simple case study. The distance learning format has a number of significant benefits: students are not restricted to a fixed times to start the course, can work through the material at their own speed and experience sampling etc on their own local streams. One disadvantage of the format is that students can be diverted from completing the courses, particularly during periods when area teams were under strength or when other duties took priority. During the early days of the course, students had little peer-support; however, this situation is now changing with most teams having at least one other trained diatomist who can demonstrate techniques such as slide preparation and help with identification problems.
After completion of the training course, students submit the results of their first three analyses for independent
audit; if these analyses met the criteria (see below), the student was deemed to be competent to perform routine analyses; otherwise, he or she was given constructive feedback on the samples and further slides were analysed and submitted until the required standard was achieved.
>
Maintenance of accreditation
Having achieved basic competence in diatom analysis and associated skills, analysts moved on to perform routine analyses. In order to maintain their accreditation, analysts have to fulfil three criteria:
Exercise their skills on a regular basis (analyse at least 30 samples per year);
Meet minimum criteria for annual ring-test slides (see below); and,
Meet minimum criteria for on-going training (see below)
In addition, one copy of each slide analysed should be submitted to a national herbarium; however, to date, this only takes place in Scotland, where slides are archived at the Royal Botanic Gardens, Edinburgh.
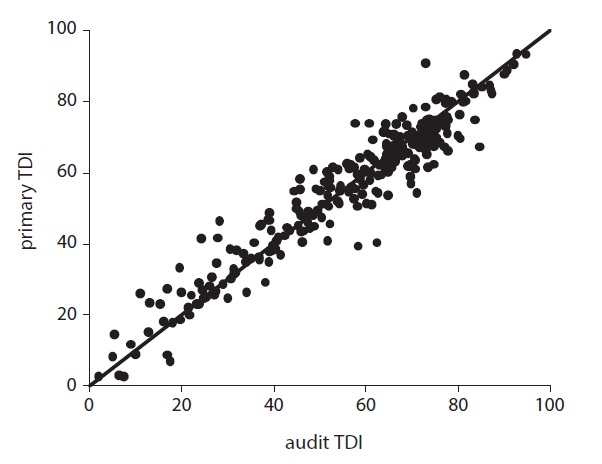
The approach to quality assurance described below rests on an understanding of the amount of natural variation that would be encountered if all sources of methodological error were minimised. Initial estimates were based on small-scale studies performed by experienced analysts; however, operation of the QA scheme over the past four years has provided us with a larger dataset of samples that have been both analysed and audited, allow-ing the scale of variation under “real world” conditions to be assessed (Fig. 1). Assuming the (generally more experienced) auditor to represent the “true” TDI, we should expect the primary analysis to fall within two standard deviations of a normalised distribution based on these data, which equates to ± 8 TDI units. This value, then, represents the limit of acceptable variation for routine diatom analyses.
The throughput of diatom samples in the UK is insufficient to permit a full quality control procedure to be adopted, as is the case for invertebrate analyses in the UK (Dines & Murray-Bligh 2000); however, within the Scottish Environmental Protection Agency, at least 5% of all slides analysed are audited internally. Slides for this audit are selected at random and analysed by other accredited diatomists within the organisation. If the TDI result is within the error estimates and the WFD classification remains unchanged, no action is required. If not, the sample is sent for external audit and constructive feedback is passed to the analysts.
>
The UK/Ireland diatom ring-test
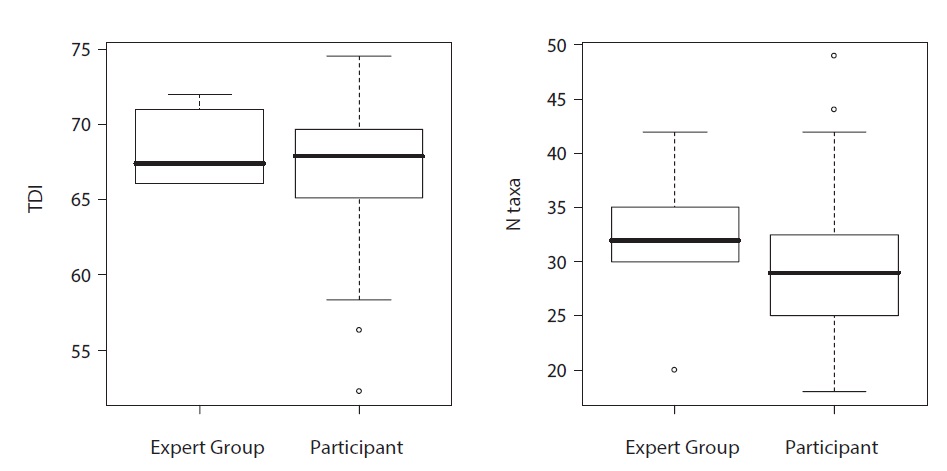
All accredited analysts are required to participate in an annual ring-test. Five slides per year are sent out to analysts participating in the scheme, each of whom then analyses the slide following a standard protocol and returns their results to the organiser, who computes TDIs and summary statistics. At the same time, a group of experienced analysts (termed the “expert panel”) also analyse the slides. The mean TDI of the expert panel then forms the “target” which other participants should achieve, whilst ± 2 standard deviations of their mean serves as a “warning limit”. This approach is preferred over the use of a single analyst’s result as the “target” (e.g., Kahlert et al. 2009) as it means that the target reflects a consensus opinion and also means that the warning limits reflect the natural variability of the sample. The ring tests also help highlight genera and species where more taxonomic clarity is required.
The ring-test scheme quickly evolved from a formal analytical quality control exercise to one of reflective practice, defined as “a set of abilities and skills, to indicate the taking of a critical stance, an orientation to problem solving or state of mind” (Moon 1999). This approach is used widely in professional development and is more appropriate to biological analyses, which relies on the
experience and intuition of the analyst, than the quality control procedures used in chemistry, where procedure and instrumentation are the main variables. To this end, the main taxa on each slide were photographed and compiled into a report so that participants could compare their own results with the expert’s results.
Results are assessed using the TDI or LTDI, as results can then be interpreted directly in terms of implications for ecological status assessments. A drawback of this approach is that it is theoretically possible to obtain a satisfactory result from a ring test by mistaking a taxon for another which has the same sensitivity value in the TDI or LTDI. Use of Bray-Curtis similarity has also been considered (Kelly 2001) and this or a similar statistic may be included in the future to increase reliability.
An example of the ring test in action is given in Fig. 2. This sample was dominated by small naviculoid diatoms, which many analysts found challenging to name. The ring test provides reassurance for most analysts that, despite their struggles, their analyses were similar to those of experts whilst, at the same time, the report provided guidance on identification of the most abundant taxa found. A follow-up workshop on the taxonomy of small naviculoids was also organised as a result. The scheme currently has 73 participants: 60 from UK agencies, one from the Irish Environment Protection Agency and 12 from universities, research institutes and consultancies.
The basic training provides a foundation on which routine analyses can be based whilst exercising their skills on a regular basis enables analysts to encounter more taxa and become more confident in the use of taxonomic tools. However, it is important that this self-directed learning is put into context by some more structured activities. It is also important that analysts are aware of on-going research in diatom taxonomy and ecology. In order to maintain their accreditation, analysts also have to demonstrate a minimum of two days on-going training per year in order to maintain their accreditation, ideally split between structured and unstructured activities.
An ongoing series of taxonomic workshops, focussed on genera and species groups which cause particular problems, provide a structured on-going training activity. These include a mixture of practical activities and talks covering new developments in tools and recent advances in diatom taxonomy and ecology. Experienced taxonomists lecture on genera that analysts find particularly challenging, after which microscopy sessions allow them to show problem specimens to the experts.
Analysts are also encouraged to attend scientific meetings and courses to develop their knowledge of diatom taxonomy and ecology, as well as more general aspects of freshwater phycology and eutrophication.
A software package, DARLEQ, was written in order to simplify calculation of the TDI, LTDI and associated estimates of uncertainty. This is a C++ program, written using Microsoft Visual Studio which reads Excel spreadsheets and calculates a range of metrics. Separate worksheets display outputs per sample and summarised by site; the latter includes associated calculations of uncertainty (Kelly et al. 2009b).
The academic literature on biological monitoring mostly describes the development and testing of indices for particular purposes and less attention has been given to the subsequent integration of these methods into organisations responsible for managing environmental quality. Whereas the initial research phase is important for establishing relationships between biology and the environment, the implementation phase is no less important as this ensures an efficient conversion of ecological data into information suitable to answer the questions raised by catchment managers. The processes described here are based less on the formal scientific process than on the gradual accretion of experience combined with tenets drawn from management science. As environmental regulation is a function of central or regional government, such steps ensure the efficient and responsible use of public money.
The implementation of diatom analyses within UK agencies represents a break with established practice insofar as diatom analysis throughout Europe is predominately the preserve of academics or specialised consultants, often with a PhD or equivalent. In the UK we have tried to break out of this paradigm and instead treat diatom analysis as one of a number of tools available to professional biologists aiming to extract key information from samples.
The methods described here forms part of a high-level nationwide surveillance monitoring to assess the condition of all water bodies in the UK. Those that fail to attain good ecological status can then be targeted for more detailed investigations, enabling pollution sources to be identified and remediation to be planned. There has been a long-term trend of improving water quality in the UK but, paradoxically, this raises the stakes for algal-based monitoring. As the quality of sewage treatment has improved, so regulatory focus has had to shift towards management of inorganic nutrients, which may derive from diffuse as well as from point sources. Although regulators need guidance on the steps necessary to ensure tangible ecological benefits, the underpinning science base is scant compared with our knowledge of many types of pollution (Page et al. 2012). Currently our knowledge of the relationship between river diatoms and water quality is based almost entirely on correlations. More work to ensure that these have secure foundations in functional ecology is needed to ensure that reliable predictions of the ecological benefits of proposed improvements can be made.
Diatom analyses, along with macrophytes surveys, also enables ecologists unfamiliar with eutrophication and nutrient management to become familiar not just with the practicalities, but also with the background science associated with these complicated issues. As time passes, some analysts move to other jobs within their agencies, taking with them the experience they have gained. Thus, diatom analysis is not just a means of fulfilling short-term data needs; it also provides a portal through which knowledge can diffuse throughout organisations as they adapt to new regulatory challenges.