The antimicrobial activity of plant natural compounds has been reviewed a number of times. Different aspects such as phytochemical diversity, involvement in mechanisms of resistances and constitutive have been extensively analyzed. The phytochemical diversity of antimicrobial compounds include terpenoids, saponins, phenolics and phenylpropanoids (Bonanomi et al., 2009). For a long time plants have played a very important role for human life. Nowadays, the use of plants as a way of treatment is still very important for human beings (Kultur, 2007). Many plants also play an important role as aromatic herbs and spices, and they have been found to have antimicrobial activity (Yang et al., 1995) and antioxidant activity (Rim et al., 2000). It is a Korean custom to steam rice cake with
The needles of different species of the genus
Phenolic compounds are widely distributed as secondary metabolites of plants as well as some edible plants (Hagerman et al., 1998; Soong and Barlow, 2004). In recent years, polyphenols have received a great deal of attention, due to their diverse biological functions (Xia et al., 2010). Phenolic compounds were found to have effect on antioxidative and antimicrobial activity (Lee et al., 2005; Ribeiro et al., 2008). The mechanisms are thought to be responsible for phenolic toxicity to microorganisms including adsorption and disruption of microbial membranes, interaction with enzymes, and metal ion deprivation (Fattouch et al., 2007).
The aim of this study was to assess the antimicrobial activity of water extracts from fresh and fallen leaves of
The fresh leaves of
The tested microorganisms included two gram-positive bacteria (
>
Extract preparation for antimicrobial activity of three Pinus species
We soaked 200 g samples of air-dried fresh leaves and fallen leaves of the three
The crude methanol extract was partitioned with 500 ml of hexane and then the top layer was concentrated (comprising the hexane fraction). The remaining layer was successively fractionated with 500 ml of diethyl ether and then ethyl acetate (forming the ether and ethyl acetate fractions). The remaining residue was the water fraction. Each fraction was concentrated
>
Determination of antimicrobial activity
Each bacterial strain was grown in a nutrient broth at 30 ℃ for 18-24 hr prior to testing and subcultured three times for another 18-24 hr. The turbidity of bacterial cell suspensions was brought to 0.3 optimal density (OD) at 660 nm by adding sterile broth and was then used for the tests. We poured 0.1 ml of the bacterial cell suspensions uniformly on nutrient broth agar plates. The paper disks containing the extract (water fraction) was carefully placed on the seeded Petri dishes. The diameters of the resulting inhibition zones were measured in mm after the cultures were incubated at 30 ℃ for 24 hr or 48 hr (Kumar, 2006). The antimicrobial activity was calculated as the net zone of inhibition estimated from the growth inhibition zone measurements (Magasneh and El-Oqlah, 1999). The minimal inhibition concentration (MIC) was determined as the lowest concentration that caused an inhibition zone.
>
Extract preparation for total phenolic content
Methanol/water (80:20 v/v, 50 ml) was mixed with airdried and powdered samples (5 g), and the phenolic substances were extracted using a vortex at 40 Hz for 3 min. The mixture was centrifuged (1200 g, 10 min) and the resultant clear solution was separated. Each extraction was conducted in duplicate. The final volume of clear supernatant was made to 10 ml with 80 % methanol and analyzed for total phenolic contents.
>
Determination of total phenolic content
Total phenolic content was determined by a slight modif?ied Folin?Denis method (Padda and Picha, 2008). A sample volume of 0.5 ml was placed in a 25 ml test tube and mixed with 8mL of distilled water followed by the addition
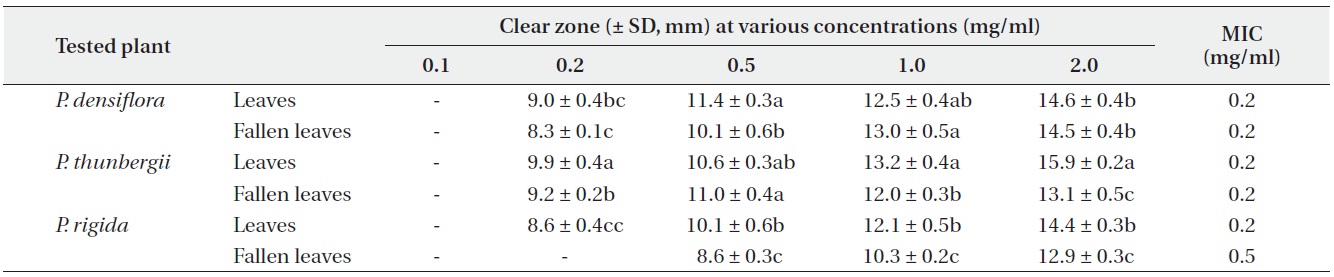
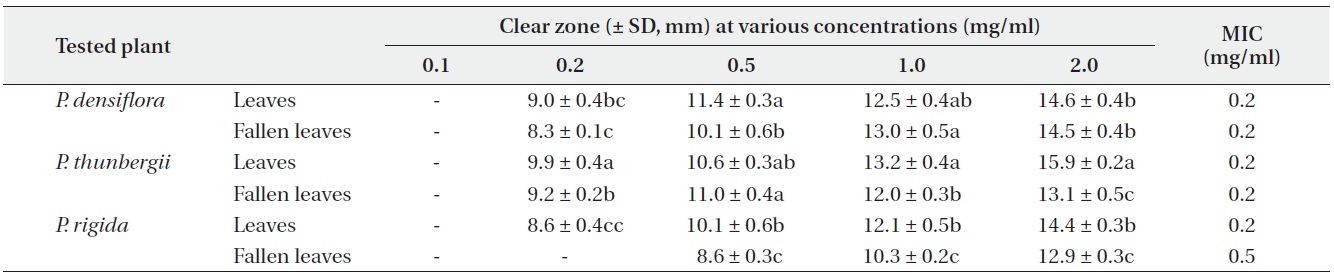
Antimicrobial activities of the water fraction of methanol extract against Staphylococcus aureus
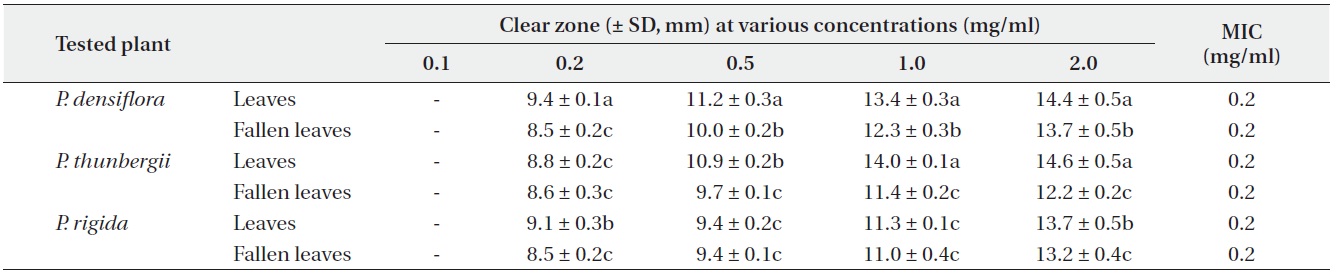
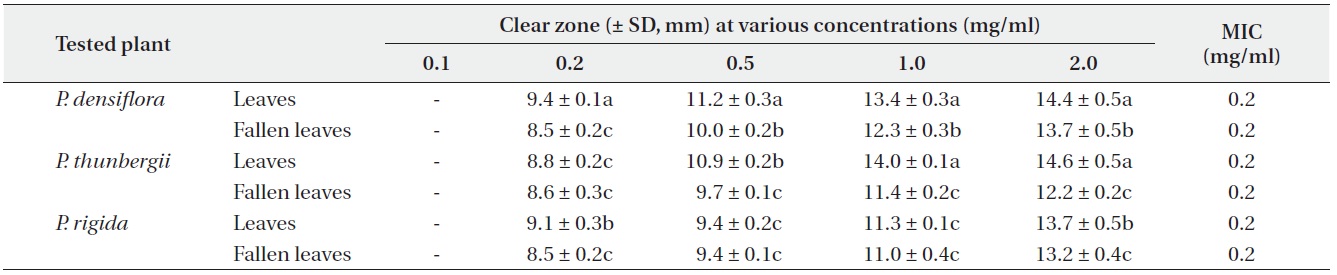
Antimicrobial activities of the water fraction of methanol extract against Bacillus subtilis
of 0.5 ml of Folin-Denis reagent. After 3 min, 1 ml of sodium carbonate (10 % in distilled water) was added and the solution was allowed to stand for 2 hr at 22℃ in darkness. The absorbance was measured at 700 nm with a UV?vis. spectrophotometer (HP-8453, USA). A standard curve with tannic acid (50-300 mg / l) was used for quantification and the total phenolic content was expressed as milligrams tannin per gram dry weight.
A randomized complete block design with three replications was applied in all the experiments. Each experiment was repeated three or four times. Statistical analysis was performed with the software program SPSS (Version 16.0). The data represent the mean ± standard deviation. The level of significance was set at
>
Antimicrobial activity of three Pinus species
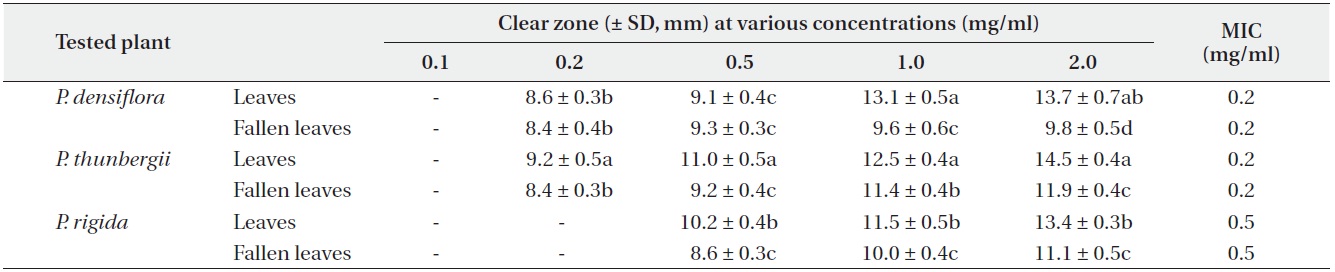
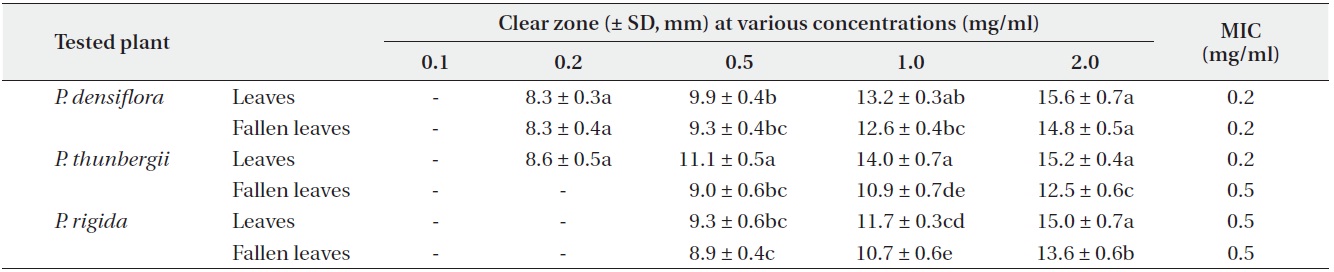
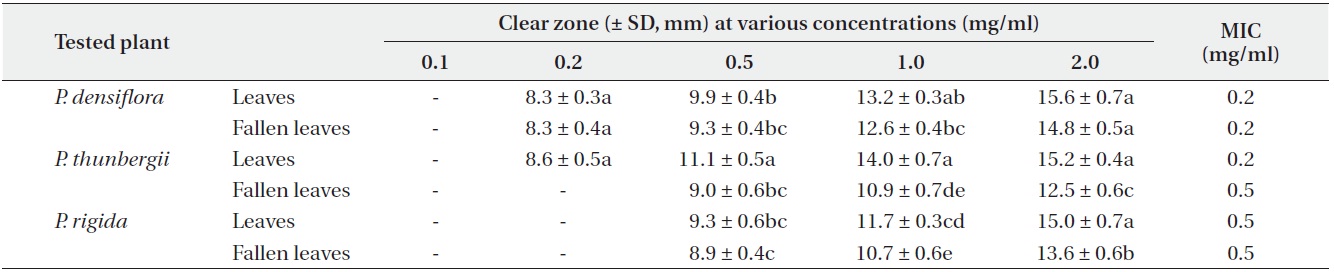
The antimicrobial activity and minimum inhibitory concentration (MIC) of water fractions of methanol extracts from fresh leaves and fallen leaves of the three
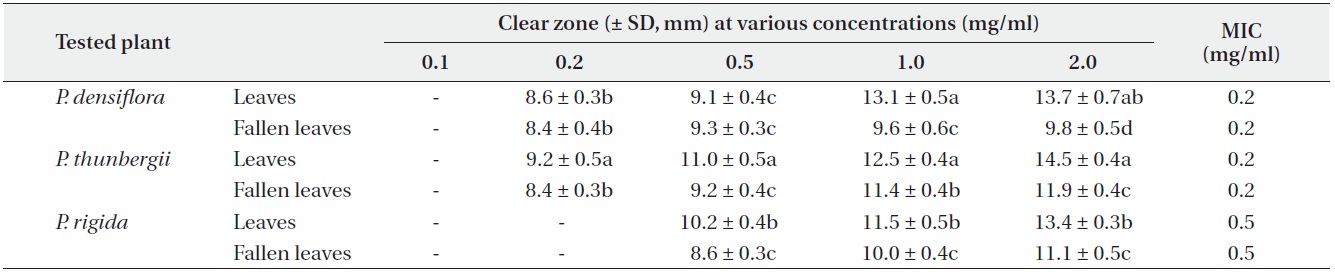
Antimicrobial activities of the water fraction of methanol extract against Escherichia coli
Antimicrobial activities of the water fraction of methanol extract against Pseudomonas flurorescens
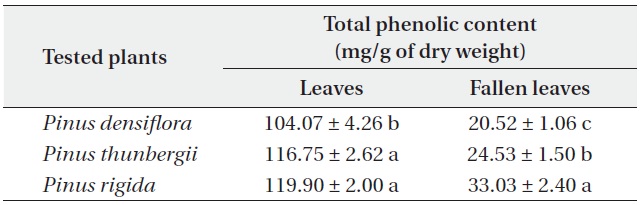
[Table 5.] Total phenolic contents (mean ± SD) of Pinus densiflora, P. thunbergii and P. rigida
Total phenolic contents (mean ± SD) of Pinus densiflora, P. thunbergii and P. rigida
The water fractions of methanol extracts from fresh leaves of
The antimicrobial activity of water fractions of methanol extracts from the three
The results from the disc diffusion method followed by measurements of minimal inhibition concentration (MIC) indicate that
>
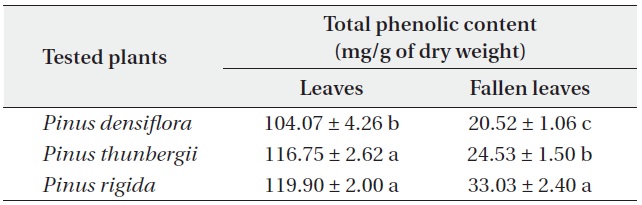
Total phenolic contents of three Pinus species
The total phenolic contents of the three
The fresh pine leaves have been used as foods and medicines in Korea (Kim et al., 2006). The antimicrobial activity of water fractions of methanol extracts from the three
Similar results have been reported antibacterial activity of
Our result revealed that increasing concentrations of the three
The results from the disc diffusion method followed by measurements of minimal inhibition concentration (MIC) indicate that
Phenolic compounds are ubiquitous in plants which collectively synthesize several thousand different chemical structures characterized by hydroxylated aromatic ring. Phenolic compounds represent the most studied phytochemicals and have been widely exploited as mode system in different areas of plant research (Boudet, 2007).
Most phenolics that display antimicrobial activity are phenolic acids or flavonoids. Phenolic acids are a major class of phenolic compounds occurring in a diverse range of plants (Wojdylo et al., 2007). Among the phenolic compounds, protocatechuic acid was the major phenolic compounds in
Antimicrobial activities and total phenolic contents of fresh leaves were higher than those of fallen leaves. The antimicrobial activity and the total phenolic content of the three
In this study, the water extracts of fresh leaves from the three