Chemically modified graphene, which is produced through the oxidation of highly oriented pyrolytic graphite, is composed of typical two-dimensional (2D) particles and is attracting a great deal of interest in colloidal sciences [1-14]. The solution processability of graphene oxide (GO) permits application of GO to substrates via spin-coating, spray-casting, drop-casting, or inkjet printing for large-scale production of graphene electronic circuits. Subsequent reduction of GO nanoplatelets can proceed through deoxygenation by chemical reduction or by thermal routes for recovery of their electrical properties.
GO nanoplatelets can be generated by chemical exfoliation of graphite with strong acid treatment, a technique introduced by Hummers and Brodie. Epoxy and hydroxyl groups are formed in the basal plane and carbonyl and carboxylic acid groups in the edge sites [15]. Due to the presence of hydrophilic functional groups, GO nanoplatelets are easily dispersed and exfoliated in an aqueous solution or organic solvent by sonication or shearing with a homogenizer. The oxidation and exfoliation processes produce diverse sizes of GO nanoplatelets. The electrical conductivities of chemically-modified graphene nanoplatelets depend on the defect level and the sheet size [12]. Furthermore, small-sized GO nanoplatelets can be used as a p-type dopant and surface modifier of a single-walled carbon nanotube electrode [16]. Therefore, the method used to separate the small-sized or large-sized graphene nanoplatelets is an important issue for allowing diverse application of chemically-modified graphene.
Here, we report a facile method for size-sorting of GO nanoplatelets dispersed in various solvents by a simple centrifugal method. Graphite oxide was exfoliated into GO in various solvent media. We found that various GO sizes can be obtained depending on the centrifugal process and the dispersion solvent.
GO nanoplatelets were prepared via the exfoliation of graphite oxide powder, which was produced from natural graphite (Alfa Aesar, 99.999% purity, -200 mesh) using a modified Hummers method. Briefly, 20 g of graphite and 460 mL of H2SO4 were mixed in a flask. Then, 60 g of KMnO4 was added slowly, over a period of approximately 1 h. Stirring was continued for 2 h in an ice-water bath. After the mixture was stirred vigorously for 3 days at room temperature, 920 mL of deionized water was added, and stirring proceeded for 10 min in an icewater bath. Fifty milliliters of H2O2 (30 wt% aqueous solution) was then added, and the mixture was stirred for 2 h at room temperature. The resulting mixture was precipitated and filtered to obtain the graphite oxide powder. The graphite oxide powder was dispersed in water by magnetic stirring, and the highly functionalized graphite oxide with carboxylic acid groups was removed by centrifugation of a 2 g/L aqueous GO solution. The sediment graphite oxide solution was free-dried and redispersed in deionized water, alkali solution (pH~11, adjusted by potassium hydroxide [KOH]), and dimethylformamide (DMF) to a concentration of 500 mg/L by bath sonication for 1 h to exfoliate the graphite oxide into GO nanoplatelets. The GO nanoplatelets were sorted using centrifugation at 10 000 rpm for 1 h, and the supernatant solution was then decanted. The sediment was redispersed in the used solvents to the same volume, and the centrifugation and decanting steps were repeated to prepare size-sorted GO nanoplatelets. The supernatant solution was concentrated and freeze-dried to obtain a powder for characterization.
Images of the resulting films were obtained using scanning electron microscopy (SEM, HITACHI S4800). The structural characteristics of the reduced GO (RGO) sheets were investigated by a confocal Raman spectrometer (NTEGRA SPECTRA, NT-MDT) with an excitation wavelength of 532 nm. To confirm the change in the carbon to oxygen atomic ratio in the functional groups of the films after reduction by hydrazine molecules synthesized in-situ, an X-ray photoelectron spectroscopy (XPS) analysis was conducted using a Multilab2000 (Thermo VG Scientific Inc.) spectrometer with monochromatized Al K X-ray radiation as the X-ray excitation source. A Fourier infrared (FTIR) spectroscopy (JASCO, 4200UP) analysis was performed to assign the functional group of the GO nanoplatelets.
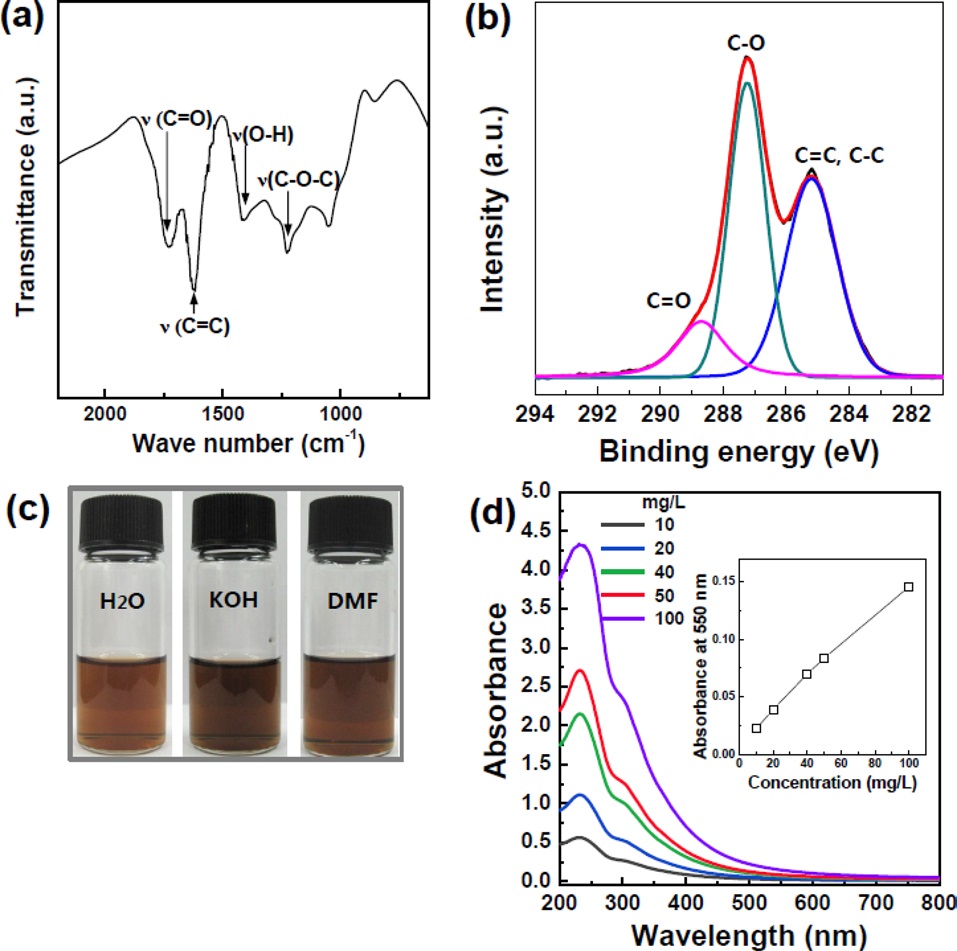
GO nanoplatelets were prepared by exfoliation of graphite oxide produced by the modified Hummers method using pure graphite (with an average size of 70 μm from natural graphite powder). Figs. 1a and b show FTIR and XPS spectra of graphite oxide, where epoxy, hydroxyl, and carbonyl groups have been assigned. Prior to the sorting process, the highly carboxylated graphite oxide (which was highly dispersible in water) was removed
by decanting the supernatant during the washing process; the carboxylated large-sized GO nanoplatelets would not have been separated from the small-sized GO nanosheets during centrifugation. A homogeneous colloidal suspension of GO nanoplatelets was prepared in deionized water (H2O), alkali solution (KOH), and DMF at a concentration of 500 mg/L by bath sonication of a graphite oxide solution for 1h (Fig. 1c). Fig. 1d shows the UV absorbance spectra of GO dispersions as a function of the GO concentration, clearly showing a stable dispersion of GO nanoplatelets in solvents. Because of their 2D, open structure, GO nanoplatelets can be cut into small-sized nanoplatelets during exfoliation by sonication [12]. The GO size distribution can therefore be widened by increasing the sonication power and time during the exfoliation of graphite oxide. The average size of the unsorted GO exceeded one micrometer. Importantly, we attempted to sort the GO nanoplatelets according to size with a GO suspension by using a centrifugal method.
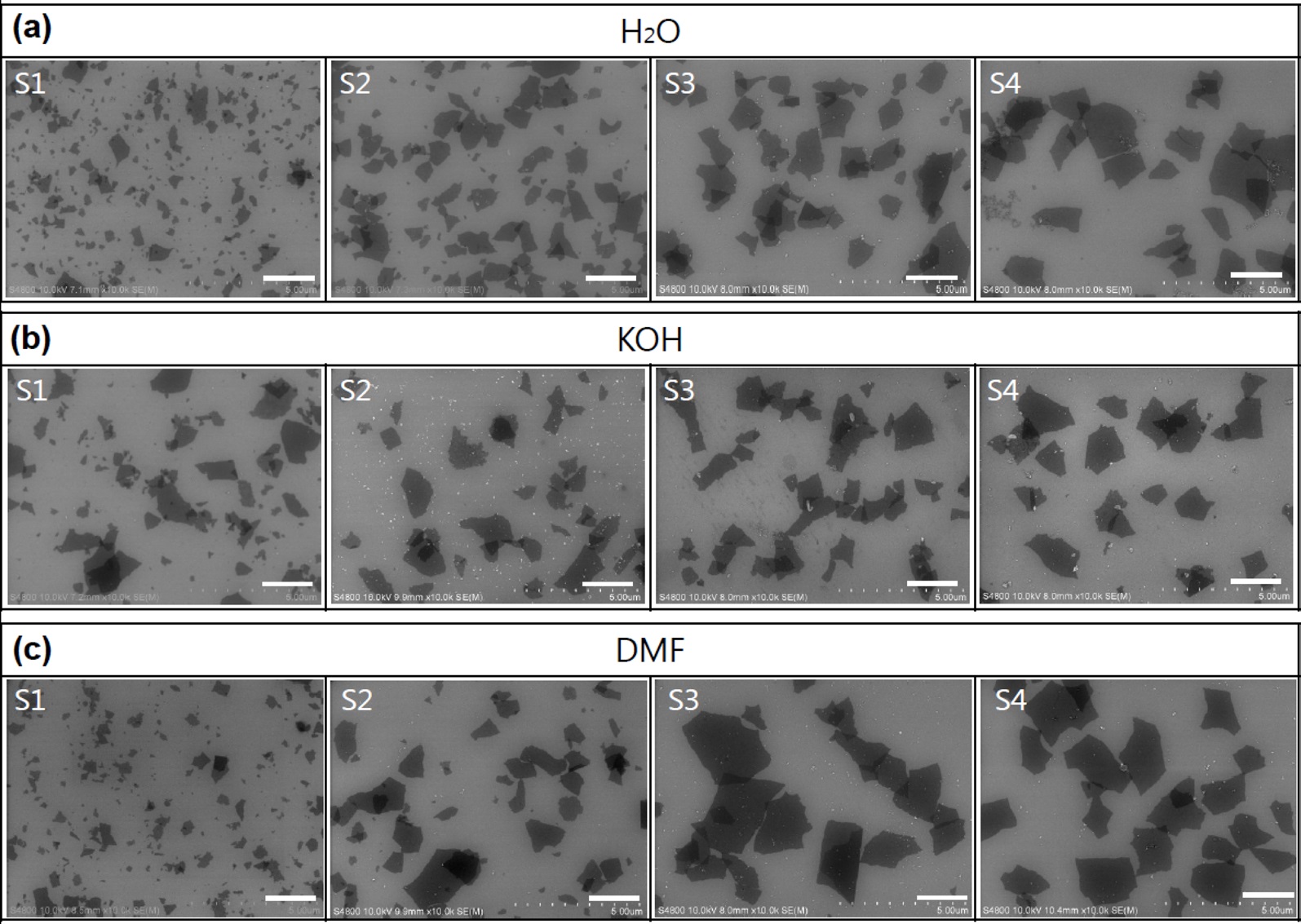
The GO dispersions in H2O, KOH, and DMF were centrifuged at 10 000 rpm for 1 h. The first supernatant solution was decanted and is denoted here as S1. The sediment was redispersed in each solvent by sonicating the suspension for 5 min (such a short time was used to minimize damage), and the solution was then centrifuged at 10 000 rpm for 1 h to obtain a second supernatant (S2). The third (S3) and fourth (S4) supernatant solutions were prepared using the same procedure. Fig. 2 shows the obtained supernatant solutions, which are light brown in color. Further centrifugation produced a lighter brown supernatant solution. The centrifuged supernatant solutions had different GO concentrations. Comparing S1 solutions in different solvents, S1 from H2O shows a darker brown color than S1 solutions from DMF and KOH solutions. This indicates that the GO nanoplatelets that are slightly reduced by the dispersion solvent are easily precipitated by the centrifugal process. SEM images
of the sorted GO nanosheets, as shown in Fig. 3, revealed that S1 contained small-sized GO (below 1 μm), and the size increased to several micrometers upon further centrifugation. The size distribution could be controlled by varying the centrifugal force and time, with a higher centrifugal force resulting in smaller GO nanoplatelets. However, in the case of the KOH solution, the sizes of separated GO nanoplatelets from S1 to S4 were not distinguishable, in contrast to H2O and the DMF solution.
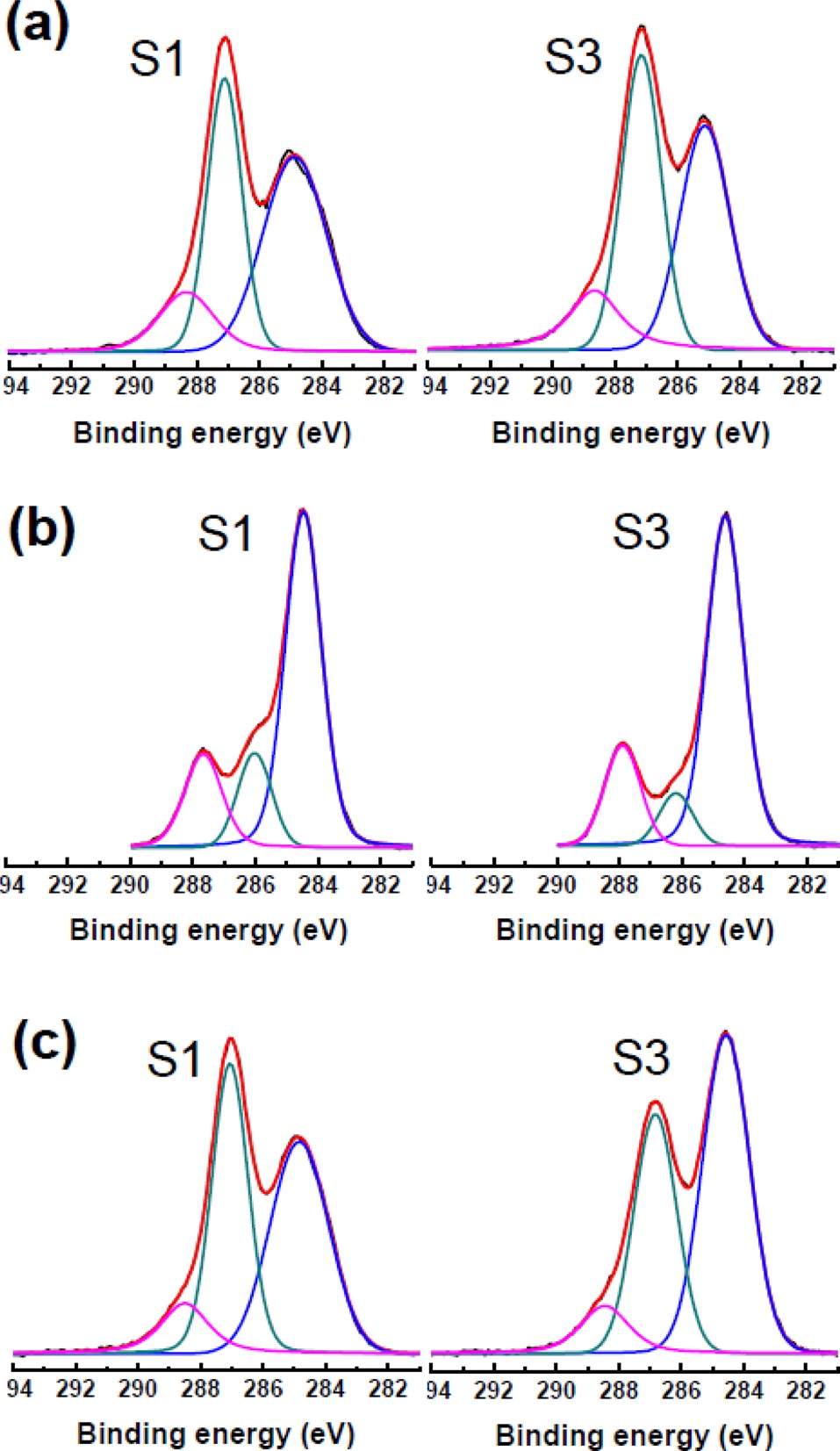
It is worth noting that the surface chemistry of the GO nanoplatelets was significantly influenced by the solvents used to disperse them. DMF, alcohol or alkali solution can reduce GO nanoplatelets in solution depending on the temperature and time [17-19]. Moderate heating during exfoliation of graphite oxide by using a sonicator or a homogenizer can reduce the exfoliated GO nanoplatelets. In the case of alkaline solution, the deoxygenation reaction depends on the pH?the higher the pH of the exfoliated-GO dispersion, the faster the reaction [19]. The XPS spectra of separated GO nanoplatelets are presented in Fig. 4. The C/O atomic ratio is an important parameter for evaluating the degree of oxidation of GO sheets. The C1s spectra were compared by deconvoluting each spectrum into three peaks. The C1s spectra of the unsorted GO nanoplatelets showed peaks at 284.6, 286.6, and 288.1 eV, which corresponded to C=C/C-C (51.6%), C-O (39.5%), and C=O (8.9%) chemical bonding states, respectively. Separated S1- and S3-GO nanoplatelets dispersed in H2O show a similar C/O ratio, while S-3 GO in DMF shows more reduced characteristics. Importantly, from the alkaline solution, the COOH groups, among several different oxygenated groups, were dominant in the separated GO nanoplatelets in terms of relative percentage. This is due to the stabilization of the carboxylic acid group by potassium ions. This means that the highly
negative charged GO nanoplatelets can be selectively separated by the centrifugal method.
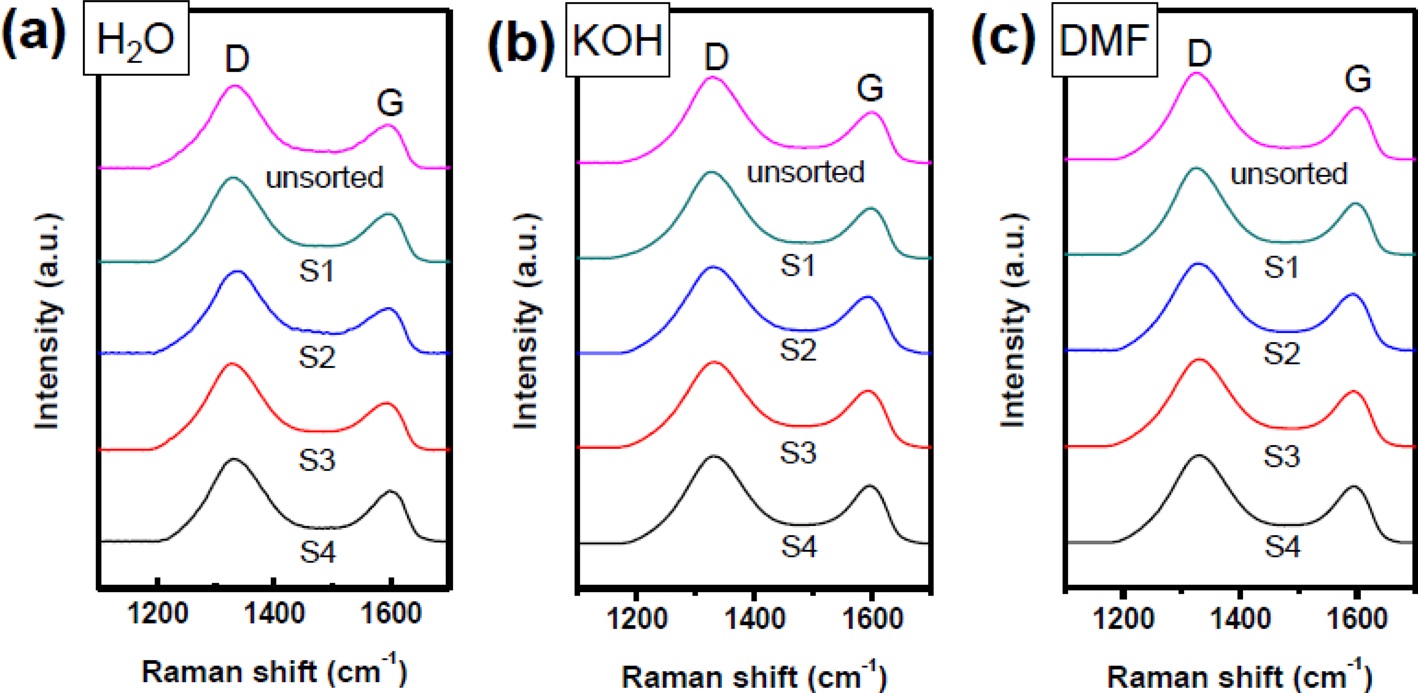
The G-band in Raman spectroscopy is active in sp2-hybridized carbon-based materials, while the D-band is activated when defects participate in double resonance Raman scattering near the K point of the Brillouin zone. Thus, the D- to G-band peak intensity ratio, ID/IG, is often used to indicate defects in a given structure. Fig. 5 shows Raman spectra of unsorted GO and separated GO nanoplatelets. It is worth noting that the D band intensities were not substantially different from S1 to S4 in all samples. These results indicate that defects in the basal plane are not significantly different from S1 to S4. Moreover, defects caused by edge boundaries were not observed in our samples, in contrast to the decreased ID/IG observed/reported for ultra-large GO nanoplatelets (>100 μm2) [20].
In this study, we systematically investigated the effects of solvents on the size-sorting of GO nanoplatelets by a centrifugal method. To do this, we first oxidized graphite by the Hummers method and separated the highly oxidized graphite oxide. The graphite oxide was then exfoliated into GO nanoplatelets in distilled water, alkali solution, or DMF for the size sorting process. Significantly, we found that distilled water was the most efficient medium to separate the small-sized GO nanoplatelets. Meanwhile, in the alkali solution, most of the GO nanoplatelets were precipitated by 10 000 rpm centrifugation because of partial reduction of GO in the alkali condition during the exfoliation and separation processes.