



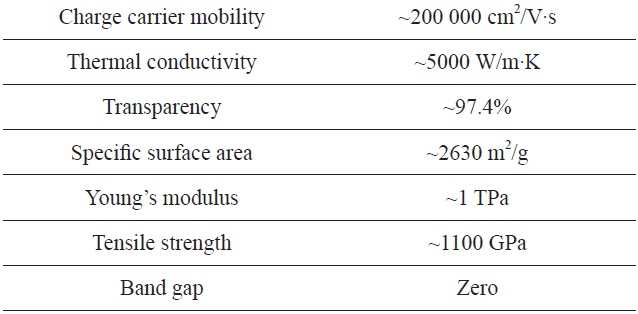
Graphene is a carbon crystal with a two-dimensional, honeycomb-lattice structure. This simple structure, however, provides exceptional physical and chemical properties including high electrical and thermal conductivities, outstanding mechanical strength and stiffness, unique optical properties, and extreme chemical stability (see Table 1) [1-4]. Graphene crystal, consisting of
The current period of active research on graphene ignited after Geim and Novoselov at Manchaster successfully peeled off a single-layer of graphene from a graphite flake in 2004. Although the first graphene was physcially exfoliated, most of the high quality graphenes used now are chemically synthesized in several different ways (e.g., oxidation and reduction, chemical vapor deposition, pyrolysis) [1-4]. While the techniques for their synthesis are few, the applications of graphenes are spread over a wide range of technologies including transparent and flexible electronics, capacitors, batteries, transistors, data storage, sensors, printable inks, barrior materials, microelectromechanical system and nanoelectromechanical system, and nanocomposites. Additional details and examples of these physical applications of graphenes can be found in several other excellent reviews of the work on graphenes [6-12]. Very recently, these graphene materials have started to assume unique roles in biological and biotechnical fields [13,14]. The early starters were cell scaffold substrates for tissue regeneration, carriers for drug or gene delivery, detection materials for bioimaging and biosensors, and neural interfaces.
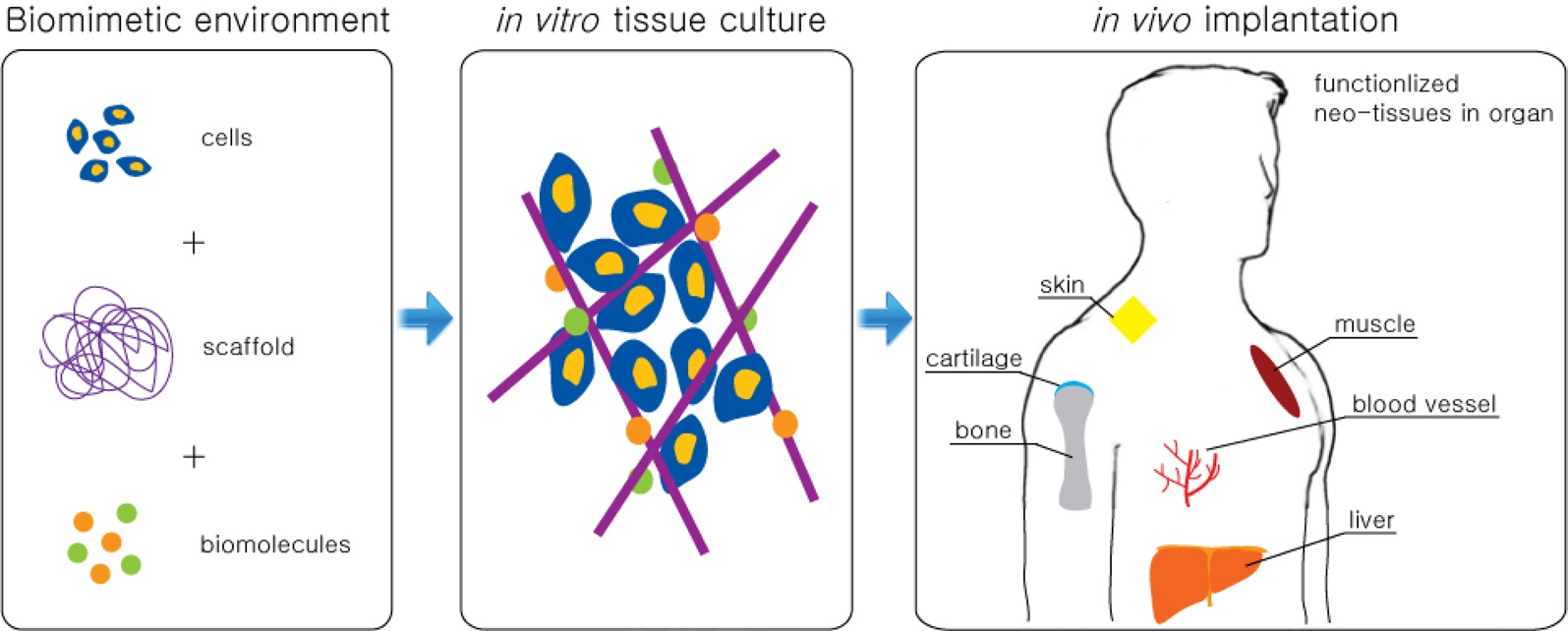
One common tissue engineering technique is to regenerate damaged tissues with functional neo-tissues made from 1) biocompatible materials and scaffolds, 2) viable cells, and 3) enabling bioactive components, such as protein, enzymes, and growth factors. This engineering technique can be applied to bone, cartilage, muscle, skin, blood vessels, and to
[Table 1.] Unique physical properties of graphene as materials
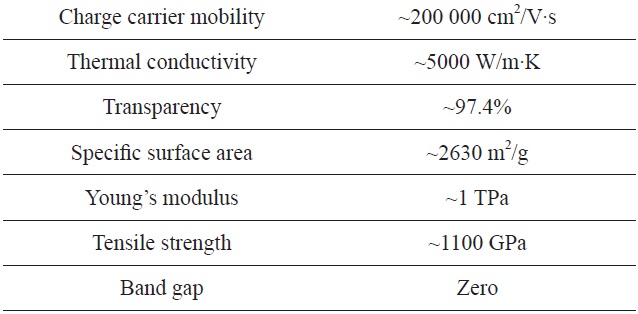
Unique physical properties of graphene as materials
most other organs (Fig. 2). Among these different therapies, all the engineering technques share some common material factors: surfaces that interface with living cells, scaffolds that guide cell growth and modulation, and carriers to deliver bioactive molecules. Furthermore, in combination with stem cell (SCs) technology, materials with qualities appropriate for directing differentiation of SCs has now become much more critical for tissue engineering. Graphene is one of the most versatile nanomaterials currently available because of its exceptional properties. Therefore, we strongly propose that graphenes are also a strong
material candidates for some, if not all, tissue engineering applications. These might include such as bone regneration scaffolds, drug delivery carriers, and neural interfaces, after adjustment of the biocompatible properties of graphenes.
Major biological and environmental safety concerns exist, in relation to the use of graphene, because the potential for its uses have exploded in rather a short time. Some have focused attention on the idea that graphenes (similar to concerns for carbon nanotubes) could be toxic if accumulated in high concentrations in the lungs [14-16]. However, it appears that proper attention to surface functionalization and control of the size of graphene products, could dramatically reduce the potential for toxicity in animals and humans. Yang et al. [17] has reported that polyethylene glycol (PEG)-functionalized graphene, which was used to treat mice, did not obviously affect liver and kidney functions over three months. Thus, management of potential for toxicity became our first prerequisite to utilizing the superior properties of graphene for biotech applications. In this review, we will discuss the recent pioneering works using graphenes in the field of bioengineering, particularly for biomedical tissue engineering, which includes cell modulation, tissue scaffolds, drug delivery, and neural interfaces.
2. Applications of Graphene for Cell Scaffolds to Control Cell Growth
2.1. Utilization of the mechanical strength and stiffness of graphene
One powerful tissue engineering approach is to modulate differentiation and growth behaviors of, and to control the attachment and sitting patterns, of cells. The exceptional physical properties of graphene certainly have huge potential, when combined with sophisticated derivatives and composites, to provide functional, biologically active surfaces. Thus, the first step towards versatile biomedical materials is to ensure the biocompatibility of various types of graphene by testing their roles in cellular processes, such as cell viability, adhesion, and spreading. Furthermore, because it is mechanically stiff and strong, several types of osteoblast cells were tested with graphene as a prospect for bone regeneration or bone treatment scaffolds, at the same time as the check
for biocompatibility. Park et al. [18] showed that a hybrid paper of soft polyoxyethylene sorbitan laurate (TWEEN) and reduced GO (RGO), with the capability to create a tight, durable seal, was safe enough to be used in attaching three different mammalian cell lines (i.e., Vero cells [African green monkey kidney cells], embryonic bovine cells, and Crandell-Rees feline kidney cells [CRFK]). This TWEEN/RGO paper was not just biocompatible, but also antimicrobial, inhibiting nonspecific binding of Grampositive bacteria (Fig. 3). Kalbacova et al. [19] investigated the biocompatibility of single-layer graphenes by attaching human osteoblasts: SAOS-2 and mesenchymal stromal cells (MSCs). When compared with SiO2, the biocompatibility of graphenes was confirmed by doubled osteoblast growth from seeding. The MSCs cultivated on the graphene layer were homogenously dispersed on the surface, and exhibited spindle-shaped cell morphology, whereas those cultivated on the SiO2 substrate formed in clustered islands of polygonal cells. This allowed higher proliferation as well as higher potential for osteoblast differentiation of MSCs on the graphene, than on the SiO2 substrate. Guo et al. [20] tested the cell adhesion and the growth of breast cancer cells MCF-7, on the graphene layers decorated with artificial peroxidases and laminins. Scanning electron microscope (SEM) images indicated that graphene can greatly promote cell adhesion and pseudopodial cell configuration starting from a round shape. Furthermore, coating thin laminin over the graphene was eight times more effective for MCF-7 cell proliferation than was the pristine surface (Fig. 4). Ryoo et al. [21] compared the biocompatibility of different carbon nanomaterials (i.e., multi-walled carbon nanotubes [MWCNTs], GO, GO/MWCNT-NH2, RGO, and RGO/MWCNT-NH2) by testing the viability of a mouse fibroblast cell (NIH-3T3) on those materials over two days. Their results suggest that those carbonaceous nanomaterials are equally strong candidates as functional coating materials for artificial organ implants. Hu et al.
[22] investigated cytotoxicity and antibacterial activity of GO and RGO nanosheets. The viability of human, lung-carcinoma, A-549 epithelial cells decreased as the concentrations of GO and RGO increased. More importantly, the filter papers made from GO and RGO showed antibacterial activity for Escherichia coli. So, they suggested that the graphene based materials could be used in wider ranges of biological applications with a need for antimicrobial surfaces, without severe cytotoxicity. Another report on the A-549 cell toxicity tests of GO was published by Chang et al. [23] They performed a comprehensive study on the toxicity of GO at a cellular
level and also suggested that GO had no serious toxicity on A549 cells on exposure, while it could lead to dose-dependent cellular oxidative stress at very high GO concentrations.
2.2. Utilization of the high electrical conductivity of graphene
Another important property of graphene is high electrical conductivity, with potential for use in building nanoscale structures. Thus, electrochemically active transduction by graphene could modulate the behavior of neural cells or neural differentiation, which may require bioelectrical signal transmissions. Park et al. [24] reported that the graphene surface facilitated neural differentiation of human neural SCs (hNSCs) rather than glial differentiation. This finding implies that the unique surface properties of graphene over glass, enhanced differentiation to neurons, probably by electrical coupling between the hNSCs and the graphene. This experiment also confirms that graphene is well suited for electrode materials in neural prosthetic devices. Li et al. [25] reported that the graphene was effective in sprouting neurites and also speeding the growth of neurites from mouse hippocampal neural cells. Both the average number of neurites per cell, and the length of the cells were greater on the graphene substrate than on the tissue culture polystyrene (TCPS) after 7 days. Even in a GAP-43 immunofluorescence staining test, the graphene group showed brighter fluorescence compared to the TCPS group, which implies healthier neural cells on the graphene.
2.3. Utilization of the complex physical properties of graphene
Graphene polymer composites were also tested for cell modulations with structural scaffolding functions. Sayyar et al. [26] made covalently linked graphene and poly-ε-caprolactone (PCL) composites with varied mechanical and electrical properties. The biocompatibility of these graphene/PCL composites was tested by cell viability tests with a mouse fibroblast cell line (L929), mouse myoblast cells (C2C12), and a pheochromocytoma cell line of rat adrenal medulla (PC12). In the test, L929 cell density increased approximately eight times above the seeding density on all tested graphene composite materials, which indicates the possibility of controlling physical properties of graphene composites independently without affecting cell proliferation or cell growth. Fan et al. [27] fabricated graphene/chitosan (CS) composite films by a solution- casting method. The elastic modulus of the CS composites increased over 200% by adding exfoliated graphene dispersions. The fabrication process of the graphene/CS composites for biological applications was simplified by skipping additional metal purification steps, because graphene could be synthesized without metallic impurities, unlike CNT reinforcements. Thus, the processed graphene composites showed acceptable biocompatibility
in a limited range of graphene content.
One of the most effective techniques to reduce mechanical stiffness of a material is to make porous gel structures. Thus, graphene hydrogels are frequently used as tissue scaffolding materials. Lim et al. [28] made a three dimensional graphene hydrogel using a hydrothermal method. As expected, the porous structure of the hydrogels could be shaped by the stiffness of hydrogel materials. Thus, with the larger the graphene, they could make hydrogels with higher porosity. To confirm the biocompatibility of the graphene hydrogel scaffolds, they seeded MG63
cells; then monitored their growth for seven days. The proliferation rate of MG63 cells decreased during the first to fifth days, but began to increase at seven days. The morphology of MG63 cell spreading revealed that the cells were well adapted to the graphene hydrogel substrates. Yang et al. [29] also made porous GO-poly (propylene carbonate, PPC) nanocomposite foams using a critical point drying technique. Then, they tested the composite foams with L929 cells. The proliferation level of L929 cells varied from 91% on the 1st day of culture to 75% on the 5th day, indicating that the processed GO/PPC foams are not very toxic.
SCs are one of the most powerful tools in tissue engineering because they have the potential to be differentiated into any cell type desired, if perfectly controlled cell environments are provided. To this end, graphene can also be a versatile player because of its many unique properties. Chen et al. [30] reported that graphene materials could be used to modulate the differentiation potentials of induced pluripotent SCs (iPSCs) of mice. When GO and graphene were compared for these iPSC cultures, the iPSCs on the GO surface proliferated faster, and those cells on the graphene maintained their undifferentiated state longer. The iPSCs on both graphene and GO surfaces spontaneously differentiated into ectodermal and mesodermal lineages; however, graphene suppressed the iPSC differentiation towards the endodermal lineage, whereas GO improved endodermal differentiation. Wang et al. [31] reported that a fluorinated graphene
(FG) induced higher SC proliferation and strong polarization. They compared a partially FG, fully FG and a pure graphene as a scaffold for MSCs. The MSCs showed a three-fold increase in cell density and more elongation on FG. Furthermore, randomly seeded MSCs exhibited preferential attachment on the FG strips, and more elongated morphology into the FG microchannels.
Ku and Park [32] investigated the behavior of myoblasts on graphene-based materials. C2C12 cells were seeded on uncoated, GO-coated and RGO-coated glass. Cells on the GOcoated glass showed the highest elongation-aspect ratio and the highest proliferation rate of all the myoblasts. Furthermore, the graphene derivatives stimulated myoblast fusion as well as myotube maturation, and enhanced myogenic differentiation to multinucleate myotubes. Sebaa et al. [33] demonstrated the viability and proliferation of human embryonic SCs (hESCs) on graphene and MWCNT-graphene hybrid substrates. Preferential use of the graphene, or the MWCNT-graphene hybrid, did not affect the viability of H9 hESCs. Furthermore, there was no significant difference in pluripotency on either substrate for nine days. Park et al. [34] suggested that graphene coating of SiO2/Si substrate caused no apparent difference in cell viability of human neuroblastoma (SH-SY5Y) after three days incubation. The cell viability was 84% on graphene-coated and uncoated glass compared to cell culture polystyrene. The fluorescence results using the dyes, Calcein AM and Hoechst 33342, also showed slightly more green-staining on the graphene-coated glass, indicating preferential cell viability.
3. Applications for Drug Delivery
Drug delivery systems (DDS) have been devised to minimize the side effects of bioactive drugs by restricting their functions to only the desired sites. Such systems have also been used to prolong medicinal effects or treatments. The current challenges in DDS research are developing a smart delivery system for recognized targets, which is called ‘targeted drug delivery,’ and a sustained and responsive release system for the drugs. Realization of an optimal release system, will require controlling complex transport and surface phenomena (e.g., diffusion, degradation, swelling, release profiles, and adsorption of DDS elements) [35,36].
In this context, we project that graphene will find vast opportunities as a DDS carrier because of its exceptional versatility and functionality. One proven strategic route is that water insoluble hydrophobic bioactive agents, can attached to the surface of a graphene by physical bonding in the forms of hydrophobic, van der Waals, or π?π stacking interactions. Graphenes with bioagents may be further modified to be soluble in aqueous conditions by grafting water-soluble molecules onto them. Thus, the resulting graphene complexes could facilitate the overall efficacy of the drug. Liu et al. [37] showed that a GO-PEG complex can be physically decorated with a water insoluble aromatic SN38 (7-ethyl-10-hydroxy-camptothecin), thus creating reservoir sites for hydrophobic drugs. Because of the extremely hydrophobic nature of graphene crystal surfaces, SN38 adhered well to the complexes, and the whole complexes showed controlled release of hydrophobic drugs in physiological serum solutions. These GO-DDS complexes would be useful for broad ranges of biomedical applications because of their safety and non-cytotoxicity, which were confirmed by in vitro cell experiments. Yang et al. [38] also demonstrated that the GO-Fe3O4 magnetic nanoparticle hybrids are suitable for DDS carriers. Their loading capacity of the drug, doxorubicin hydrochloride (DXR), was 1.08 mg/mg, which was much higher than all other common drug carrier materials (i.e., polymer micelles, hydrogel micro-particles, liposomes, and carbon nano-horns). These hybrid complexes
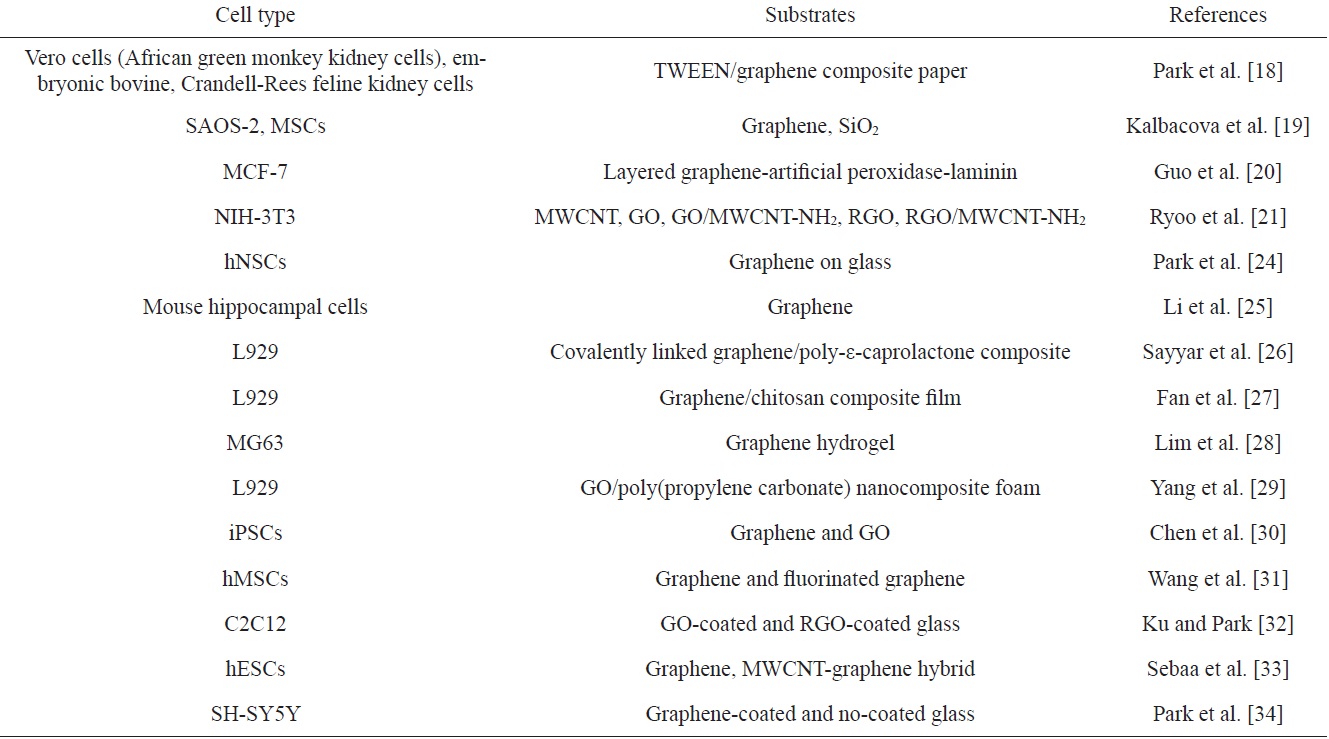
[Table 2.] Graphene-based cell modulations
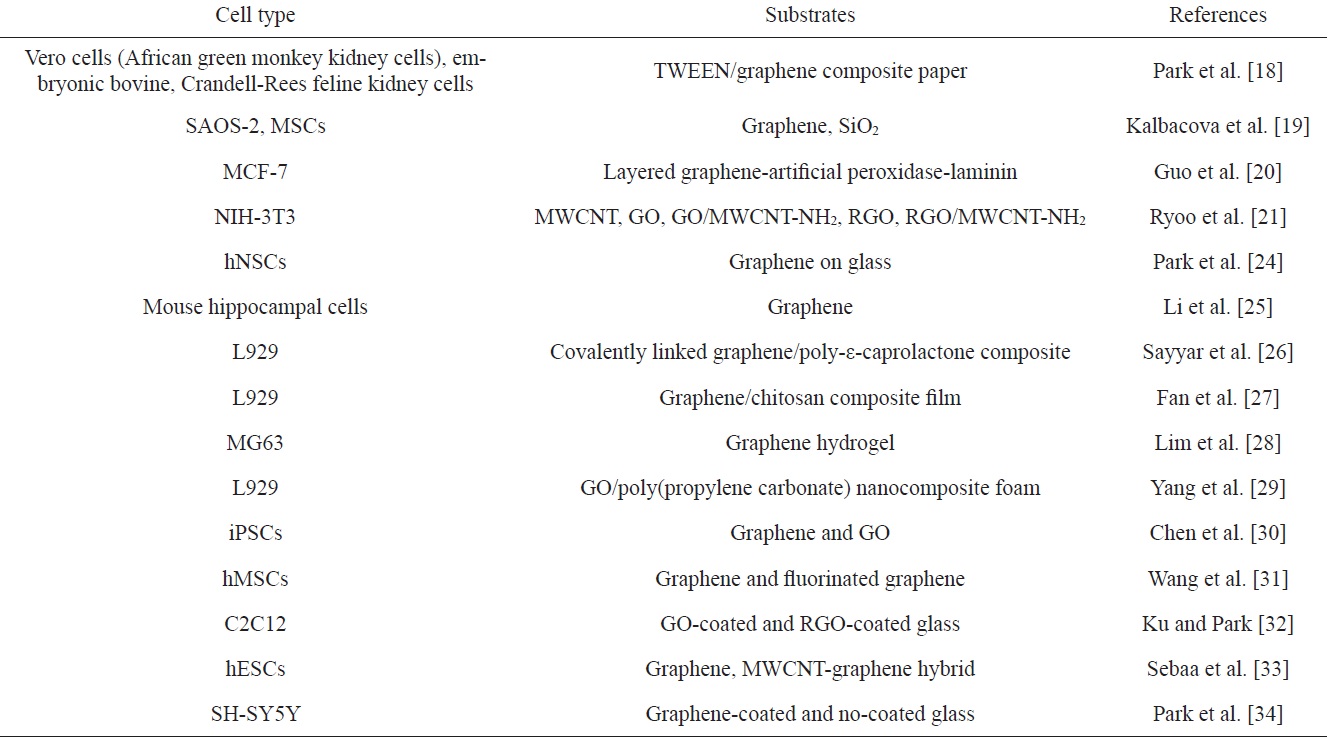
Graphene-based cell modulations
can be congregated and dispersed reversibly under different pH conditions. This pH-triggered, controlled magnetic behavior, provides a unique advantage as DDS carriers. Depan et al. [39] reported on doxorubicin (DOX) loaded GO, encapsulated with folic-acid-conjugated CS, as a drug carrier. This carrier showed better controlled, and prolonged, drug release after encapsulation, compared to without encapsulation. Furthermore, because the DOX were attached to GO by physical π?π interactions, release was highly sensitive to pH under physiological conditions.
Intrinsic biomaterials can be used to modify graphene to be more biocompatible. Liu et al. [40] reported that gelatin wrapped graphene nano-sheets (GNSs) exhibited no cytotoxicity. In acidic environments, the complexes showed fast drug release, as well as high loading capacity under other conditions. The drug delivery actions were demonstrated with complexes loaded with the anticancer drug, DOX, which killed MCF-7 cells, while other complexes without the drug did not interrupt growth of the cells. Similarly, graphene could be modified with biocompatible CS. Bao et al. [41] reported that a covalently grafted GO with CS (GO-CS), could be used as a nano-carrier of camptothecin (CPT), an anticancer drug insoluble in water. The release profile of the nano-carrier gradually increased to 17.5% for 72 h. Cell toxicity of the carrier was tested by MTT assay of human hepatic and cervical carcinoma cells (HepG2 and HeLa). While GO-CS showed no toxicity up to 100 mg/L, GO-CS-CPT showed a 50% growth inhibition concentration (IC50) at a concentration of as little as 29 μM.
4. Applications for Neural Interfaces
A neural interface is a communication system between a tissue in a body and an external electrical device, which is mostly operated by electrical signals. Because biological cells are excited by ionic potentials, the neural interfaces should relay the electrochemical signals between a stiff, dry electrode and a soft, wet tissue. The challenges here are not just showing good physical properties and performance, but also chronic biocompatibility and functional stability of the electrode [42]. The essential qualities of neural electrodes include 1) biocompatibility to delay or avoid immune responses by the body, 2) flexibility to accommodate the difference between soft tissues and rigid devices, 3) safety for the cells to prevent them being damaged, 4) chemical or electrochemical stability to bear changes within the body, and 5) selectivity and sensitivity to effectively measure electrical signals [43]. While hard, rigid or stiff materials (e.g., ceramics and metals?platinum, iridium, and gold; silicon, and indium tin oxides), are currently used for neural electrodes, softer electronic materials are now receiving increasing attention to better adapt to the difference in interfacial qualities of cells and electrodes.
Initially, conducting polymers, including polyaniline (PANI), polypyrrole (PPy) and poly(3,4-ethylenedioxythiophene (PEDOT), showed promising results with improved biocompatibility and electrochemical impedances over conventional metals [45-47]. After discovery of the limitations of functional durability of those conducting polymers after chronic implantation, carbon nanomaterials are now being investigated as new types of neural electrodes [48,49]. Thus graphene, along with other carbonaceous materials (e.g., carbon nanotubes), is emerging as a potential neural interface material. Zhou et al. [50] coated poly-ε-caprolactone, electrospun, nanofiber scaffolds with heparin modified graphenes and polyl- lysine using layer-by-layer (LBL) assembly techniques. These graphene coated composites significantly lowered sheet resistance and enhanced attachment of primary cortical neurons onto the scaffolds. By mixing and matching during LBL assembly, these electro-active components spread uniformly over the surface of the porous scaffolds, while bioactive components effectively reduced any detrimental effect to the biological neural tissue cells. Bendali et al. [51] investigated the survival ratio of primary, retinal ganglion cells on a graphene surface compared to glassy substrates. On the glassy substrates, a biocompatible peptide coating or glial cell supports, were essential to bring neural cells onto the surface. However, bare graphene was compatible enough that cell growth was successful, though the peptide coated surfaces were better than the uncoated ones. This work confirms that high quality graphene can be used to interface with cells directly, without other biomaterials. The biocompatibility of graphene can also be improved by appropriate physical treatments instead of being coated with other nonconducting biomaterials. Chen et al. [52] reported that graphene deposited electrodes on a flexible microprobe could be used as a retina prosthesis electrode. They treated the graphene surface with steam plasma in order to make the electrode hydrophilic as well as biocompatible. This treatment ultimately resulted in an improved signal-to-noise (S/N) ratio during neural recordings from the axons of a crayfish and the heart of a zebra fish. This noise may have been caused by proximate contacts between the cells and electrodes.
Graphene electrodes are excellent electrochemically functional materials, particularly for sensitive recording of biological signals. Hess et al. [53] measured an action potential directly from cardiomyocyte- like HL-1 cells using arrays of graphene-based transistors. The graphene-based solution-gated field-effect transistors (SGFETs) used in their work were sensitive enough to record selective biological signals, i.e., an S/N ratio of more than ten, which is
analogous to a state-of-the-art microelectrode array. Cohen-Karni et al. [54] also designed graphene-silicon nanowire FETs interfaced with embryonic-chicken cardiomyocytes. Their graphene-FET in contact with spontaneously beating cardiomyocyte cells, provided regularly spaced peaks with a frequency of about 1.1 Hz, and an S/N ration of more than four, for the conductance versus time measurement results.
Luo et al. [55] tested GO as a dopant of PEDOT films for neural electrodes. PEDOT-GO exhibited a sharp decrease in electrical impedance of the Pt-Ir neural electrode. As they tested the PEDOT-GO surface with primary cortical neurons, their neurites were extensively branched out, even within a day of incubation. There was no significant difference in viability between PEDOTGO and PEDOT-PSS, but the cells on PEDOT-GO showed longer neurite length than those on the PEDOT-PSS, which is a clear benefit for application to neural electrodes. Furthermore, laminin peptide could easily be grafted onto the GO so that its coating surface showed much improved neurite outgrowth from the cells.
For a few years, graphene research has exploded in some physical application fields, particularly, in flexible electronics. However, graphene research into bioengineering applications was relatively moderate until recently. As the more fascinating properties of graphene materials are revealed, and more interdisciplinary research efforts are attempted worldwide, more and more possibilities for graphene as a biomaterial are now being actively discovered. Thus, in this review article, we comprehensively surveyed recent experimental work related to tissue engineering and tissue regenerative medicine utilizing graphene or graphene derivatives.
The exceptional properties of graphenes are now being incorporated into many functional biological materials. Cell scaffolds, which modulate cell growth, sitting patterns, and differentiation, are just one example of graphene utilization towards better tissue regenerative medicine. Furthermore, functional graphenes form biocompatible complexes with various drugs that would otherwise be extremely difficult to deliver in physiological solutions. These complexes can be designed to deliver drugs to any desired site within bodily organs and tissues, because of both their safety and functionality within living biologicals. In addition, graphene, as a soft electronic material, could find distinct roles in neural interface engineering. The examples introduced in the review all corroborate the bright future of graphene for applications in biological tissue engineering.
![Examples of several different graphene forms [5].](http://oak.go.kr/repository/journal/11909/HGTSB6_2013_v14n2_63_f001.jpg)
![Scanning electron microscope images of TWEEN/RGO paper (a-c) composite confocal microscopy images of the three kinds of mammalian cells grown for 48 h on TWEEN-paper for the standard live-dead test (scale bars, 20 mm), (d) photos of a TWEEN paper sample, (e-f ) optical microscopy images of the TWEEN paper before and after treatment with mature Bacillus cereus cells. The TWEEN paper shows no bacterial attachment [18]. TWEEN: polyoxyethylene sorbitan laurate, RGO: reduced graphene oxide.](http://oak.go.kr/repository/journal/11909/HGTSB6_2013_v14n2_63_f003.jpg)
![Scanning electron microscope images of MCF-7 cells cultured for 12 h on (a) ITO/(AP)10 and (b) ITO/(graphene-AP)10?the scale bar is 5 μm for both, (c) proliferation curves of cells cultured on films with different compositions: 1) ITO/(AP)10 , 2) ITO/ (graphene)10 , 3) ITO/(graphene-AP)10 , 4) ITO/(graphene- AP)10?one laminin layer on top, and 5) ITO/(graphene-AP-laminin)10 [20]. ITO: indium tin oxide.](http://oak.go.kr/repository/journal/11909/HGTSB6_2013_v14n2_63_f004.jpg)
![Live and dead staining of NIH-3T3 cells after incubation on each substrate for 48 h?live cells are stained fluorescent green, and dead cells appear red (left side)?substrates: (i) glass; (ii) multi-walled carbon nanotube (MWCNT); (iii) graphene oxide (GO); (iv) GO/MWCNT; (v) reduced GO (RGO); and (vi) RGO/MWCNT?Scale bars all represent 100 μm. For the proliferation assay (right side), the number of cells on each substrate was evaluated at 24 and 48 h, and the percentage increase was calculated [21].](http://oak.go.kr/repository/journal/11909/HGTSB6_2013_v14n2_63_f005.jpg)
![(a) Viability of A549 cells incubated with 20 and 85 μg/mL graphene oxide (GO) nanosheets for 2 h and 24 h, (b) metabolic activity of Escherichia coli incubation with 20 and 85 μg/mL GO nanosheets at 37℃ for 2 h, (c) viability of A549 cell incubated with 20 and 85 μg/mL reduced GO (RGO) nanosheets, (d) metabolic activity of E. coli treated with 85 μg/mL GO and RGO nano-sheets [22].](http://oak.go.kr/repository/journal/11909/HGTSB6_2013_v14n2_63_f006.jpg)
![Enhanced neural-differentiation of human neural stem cells (hNSCs) on graphene films?all scale bars represent 200 μm. (a) Bright-field images of the hNSCs differentiated for three days (left), two weeks (middle), and three weeks (right), (b) bright-field (top row) and fluorescence (bottom row) images of hNSCs differentiated on glass (left) and graphene (right) after one month of differentiation?the differentiated hNSCs were immunostained with GFAP (red) for astroglial cells, TUJ1 (green) for neural cells, and DAPI (blue) for nuclei. c) Cell counts per area (0.64 mm2) on graphene and glass regions after onemonth differentiation, d) percentage of immunoreactive cells for GFAP (red) and TUJ1 (green) on glass and grapheme [24].](http://oak.go.kr/repository/journal/11909/HGTSB6_2013_v14n2_63_f007.jpg)
![Graphene-based composite scaffolds. (i) TWEEN/graphene paper [18]. (ii) Graphene hydrogels [28]. TWEEN: polyoxyethylene sorbitan laurate.](http://oak.go.kr/repository/journal/11909/HGTSB6_2013_v14n2_63_f008.jpg)
![Scanning electron microscope images of PPC/GO foams without cells (a), and cell-seeded PPC/GO foam after culturing 24 h in vitro (b, c)?the white arrows indicate the cell-to-cell communications. In vitro cell cytotoxicities of PPC-GO foams according to the MTT assay (d) for three days [29]. PPC: propylene carbonate, GO: graphene oxide.](http://oak.go.kr/repository/journal/11909/HGTSB6_2013_v14n2_63_f009.jpg)
![(a-c) Fluorescent images of the actin cytoskeleton of mesenchymal stromal cells (MSCs) cultured on graphene, partillay fluorinated grapheme (FG) and FG stained with rhodamine-phalloidin at day 7 (scale bar = 100 μm), (d) proliferation of MSCs cultured on the graphene films, showing the controlled growth of MSCs on fluorinated graphene with different coverage of fluorine, (e) MSCs preferentially attached and highly aligned on the FG strips (scale bar = 50 μm), (h) percentage of immune-reactive cells for TUJ1 and MAP2 on unpatterned and patterned FG strips [31].](http://oak.go.kr/repository/journal/11909/HGTSB6_2013_v14n2_63_f010.jpg)
![(A) aspect ratio quantification of C2C12 cells on unmodified, graphene oxide (GO)-, and reduced GO (rGO)-modified glass substrates?cells were cultured in GM for 1 day, (B) quantification of fusion index and maturation index?quantification of (C) cell area, (D) length of multinucleate myotubes [32].](http://oak.go.kr/repository/journal/11909/HGTSB6_2013_v14n2_63_f011.jpg)
![Fluorescence images of H9 human embryonic stem cells cultured on graphene, multi-walled carbon nanotube (MWCNT)-graphene hybrid, glass, control tissue culture polystyrene substrates for 9 days [33].](http://oak.go.kr/repository/journal/11909/HGTSB6_2013_v14n2_63_f012.jpg)
![Schematic illustration of a drug delivery system [37,38].](http://oak.go.kr/repository/journal/11909/HGTSB6_2013_v14n2_63_f013.jpg)
![Overview of neural electrode arrays applied to different sections of the nervous system [44].](http://oak.go.kr/repository/journal/11909/HGTSB6_2013_v14n2_63_f014.jpg)
![(a) Schematic view of a G-SGFET with a cell on the gate area, (b) optical microscopy image showing eight transistors in the central area of a GSGFET, (c) transistor current vs. electrolytic gate voltage measured in HL-1 cell on the array, and (d) transconductance vs. gate voltage for the transistor [53]. SGFET: solution- gated field-effect transistors.](http://oak.go.kr/repository/journal/11909/HGTSB6_2013_v14n2_63_f015.jpg)
![(A) Representation of the relative size of a cardiomyocyte cell interfaced to a typical graphene and silicon nanowire-FET device, (B) gate effect on graphene-FET recorded signals from cardiomyocytes?recorded traces at different applied water gate potentials [54]. FET: field-effect transistor.](http://oak.go.kr/repository/journal/11909/HGTSB6_2013_v14n2_63_f016.jpg)
![(A) Scanning electron microscope image of a neuron growing on the PEDOT/GO surface at 1 d, (B) bode and nyquist plots of the electrochemical impedance behavior of platinum iridium microwires uncoated, coated with PEDOT/GO and coated with PEDOT-GO covalently modified with p20, (C) average neurite length of cells growing on the polymer surfaces, (D) average neurite length of neurons growing on the p20 modified PEDOT-GO surfaces [55]. PEDOT: poly(3,4-ethylenedioxythiophene, GO: graphene oxide.](http://oak.go.kr/repository/journal/11909/HGTSB6_2013_v14n2_63_f017.jpg)
![Adult retinal ganglion cells on graphene either (A) bare, or (C) coated with poly-D-lysine and laminin?new-born retinal ganglion cells on graphene either (B) bare, or (D) coated with poly-D-lysine and laminin [51].](http://oak.go.kr/repository/journal/11909/HGTSB6_2013_v14n2_63_f018.jpg)