In this study, PtRu nanoparticles deposited on binary carbon supports were developed for use in direct methanol fuel cells using carbon blacks (CBs) and multi-walled carbon nanotubes (MWCNTs). The particle sizes and morphological structures of the catalysts were analyzed using X-ray diffraction and transmission electron microscopy, and the PtRu loading content was determined using an inductively coupled plasma-mass spectrometer. The electrocatalytic characteristics for methanol oxidation were evaluated by means of cyclic voltammetry with 1 M CH3OH in a 0.5 M H2SO4 solution as the electrolyte. The PtRu particle sizes and the loading level were found to be dependent on the mixing ratio of the two carbon materials. The electroactivity of the catalysts increased with an increasing MWCNT content, reaching a maximum at 30% MWCNTs, and subsequently decreased. This was attributed to the introduction of MWCNTs as a secondary support, which provided a highly accessible surface area and caused morphological changes in the carbon supports. Consequently, the PtRu nanoparticles deposited on the binary support exhibited better performance than those deposited on the single support, and the best performance was obtained when the mass ratio of CBs to MWCNTs was 70:30.
In recent years, direct methanol fuel cells (DMFCs) have gained a reputation as one of the most attractive power sources for terrestrial electrical vehicles owing to their high power density achievable at low temperatures. Their performance is nevertheless limited because of the slow kinetics of methanol electro-oxidation at the anode, catalyst poisoning, and the methanol crossover phenomenon. The poisoning of the anode catalysts by carbon monoxide, an intermediate of the anodic methanol oxidation, is one of the causes of the slow kinetics of the anode reaction, which is a major challenge that needs to be overcome to increase the catalyst efficiency and power density [1,2].
Among the precious metals, Pt is most commonly used as a catalyst because Pt shows the highest activity for electro-oxidation of methanol. However, the performance of a pure Pt catalyst is not satisfactory owing to the formation of strongly adsorbed intermediates. Poisoning of the Pt surface by CO during methanol electro-oxidation is the major reason for the slow kinetics. CO can strongly adsorb onto the surface of the pure Pt catalyst and form a Pt- CO species, which blocks the catalytic active sites. To solve this problem, advanced anode catalyst designs that are based on a bi-functional mechanism, have been suggested; in these designs, cocatalysts are used and Ru is added to the Pt catalysts. The best results have been reported for binary PtRu catalysts, because Ru easily removes CO from Pt. Therefore, binary PtRu catalysts are regarded as appropriate materials for anode catalysts of DMFCs [3,4].
Many researchers have attempted to improve the activity of the PtRu catalyst by various approaches. The electrocatalytic activity is strongly dependent on particle size, dispersibility, support materials, and surface conditions [5]. In addition, it is necessary to decrease the amount of precious metal used and the loading level on the support materials. Therefore, the support materials have been identified as an important parameter because they contribute to the overall catalyst characteristics. PtRu supported on carbon has gained considerable attention as an important catalyst for DMFC electrodes [6,7]. Carbon-based materials are widely used as support materials because of their textural, electronic, adsorption, and molecular sieving properties. Carbon blacks (CBs) have been widely used as electrocatalyst supports because of their high electric conductivity and surface area and their low cost [8,9]. Recently, a graphitic nanostructured carbon material, multi-walled nanotubes (MWCNTs), has been the object of focus as a catalyst support for DMFCs owing to its high strength, low density, and high electrical and thermal conductivity [10,11].
A single carbon support, however, may not easily facilitate control of an electrode structure to achieve high conductivity and porosity and good morphology. Sakaguchi
In this study, we prepared PtRu nanoparticles deposited on binary carbon supports (CBs and MWCNTs) as a catalyst for DMFCs. To optimize electrocatalytic activity, the CB/MWCNT supports were impregnated with PtRu nanoparticles with good dispersibility. The electrochemical characteristics of the PtRu/CB/MWCNT catalysts were examined for methanol oxidation in the DMFCs.
2.1. Materials and sample preparation
Binary carbon supports were prepared by mixing CBs (Hi Black 420B, Korea Carbon Corporation) and MWCNTs (Nano Solution Company) with different mixing ratios. The samples were designated as MWCNT 0, MWCNT 10, MWCNT 30, MWCNT 50, and MWCNT 100 according to the MWCNT weight content of 0, 10, 30, 50, and 100%, respectively. Prior to the preparation of the PtRu catalysts, the residual chemicals in the carbon materials were removed using nitric acid for 3 h. Both CBs and MWCNTs were washed several times with distilled water and dried in a vacuum oven at 110℃. The CB/MWCNT supports were suspended in distilled water and ultrasonically stirred for 30 min. Then, the metal precursors, H2PtCl6 and RuCl3 (Aldrich Co.), were dissolved in distilled water and added dropwise to the CB/MWCNT slurry. The metal precursors were prepared to contain 1:1 ratios, and the total loading of metal precursors in CB/MWCNT supports was 20 wt%. Next, a 5 M NaOH solution was added to adjust the pH of the solution. Then, HCHO, a reducing agent, was added to the solution and stirred at 85℃ for 5 h to reduce the Pt and Ru metals. The experiment was conducted under a stream of argon gas to remove organic by-products. The catalysts were then dried in a vacuum oven at 70℃ for 12 h.
PtRu loading levels of the catalyst were calculated using an ELAN 6100 inductively coupled plasma-mass spectrometer (ICP-MS). Structural properties of the catalyst were investigated using X-ray diffraction (XRD) performed using a Rigaku D/MAX 2200V/PC X-ray diffractometer with Cu Kα radiation. The 2θ angular regions between 20° and 85° were investigated at a scan rate of 5°/min. A transmission electron microscope (TEM, A JEM 2100F) was used to observe the surface properties of the catalyst. Before obtaining electron micrographs, the catalyst samples were ultrasonically dispersed in ethyl alcohol, and a drop of the resultant dispersion was deposited and dried on a Lacey carbon film suspended on a copper grid. The N2 adsorption isotherms were measured at 77 K using a Micromeritics TriStar 3000. Prior to nitrogen adsorption, the samples were treated in vacuum at 573 K for 6 h. The N2 adsorbed on the CB/MWCNT support was used to calculate the specific area using the Brunauer- Emmett-Teller (BET) method.
2.3. Electrochemical properties
The electrocatalytic behaviors of the catalyst were examined using Autolab with PGSTAT 30 (Eco Chemie, The Netherlands) cyclic voltammetry equipment. The measurements were conducted at 25℃ in a conventional three-electrode electrochemical cell system consisting of the following electrodes: a Pt wire as a counter electrode, an Ag/AgCl reference electrode, and a catalyst sample mixed with carbon paste on a glassy carbon electrode as a working electrode. 1 M CH3OH in a 0.5 M H2SO4 aqueous solution was used as the electrolyte for methanol oxidation measurements, and a 0.5 M H2SO4 aqueous solution served as the electrolyte for hydrogen oxidation measurements. The potential changed linearly from 300 to 1100 mV
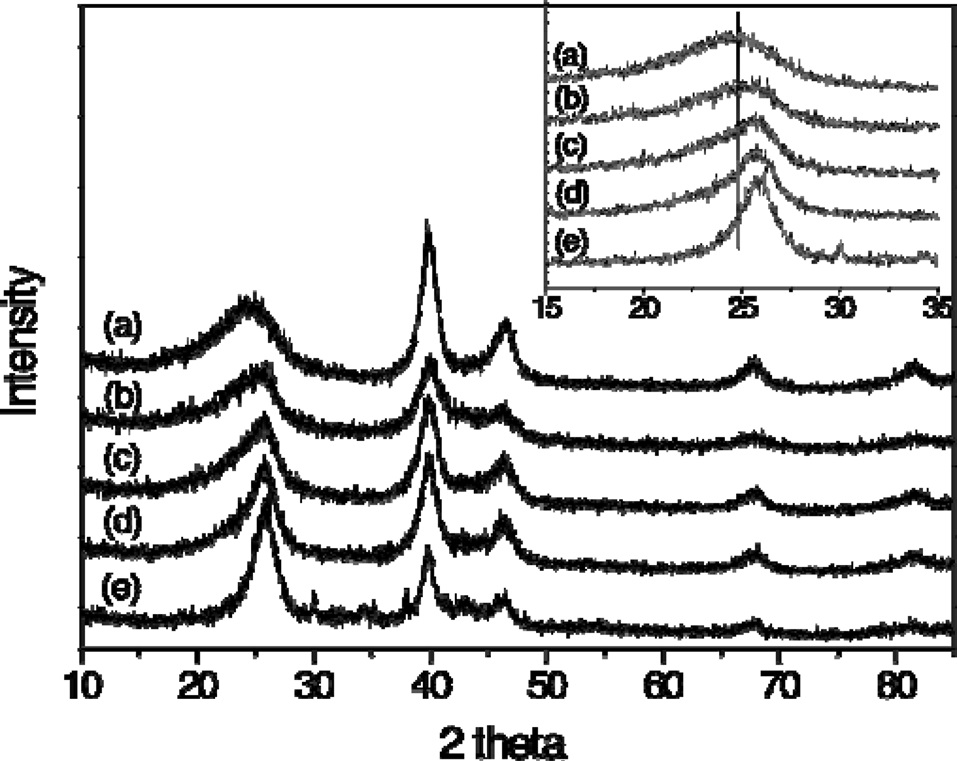
Fig. 1 shows the XRD patterns of the PtRu/CB/MWCNT catalysts. The first peaks at approximately 26° are associated with the graphite (002) peak of the CB/MWCNT supports. The intensity of 2θ at 26° gradually increased with an increasing MWCNT content, indicating that the addition of MWCNTs had an effect on the crystalline lattice of the carbon supports and that it probably corresponded to structural changes in the CBs/MWCNTs. All of them presented the important characteristic patterns of Pt face-centered cubic (FCC) crystalline structures at 2θ,
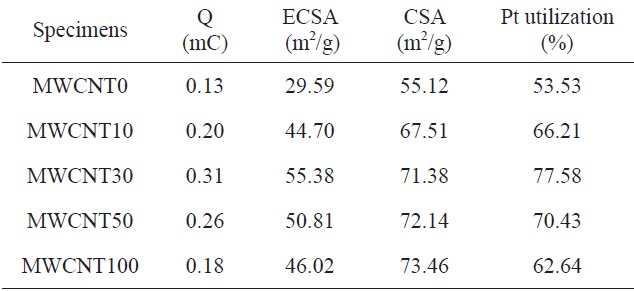
[Table 1.] The CSA, ECSA, and Pt utilization of PtRu deposited on CBs/MWCNTs
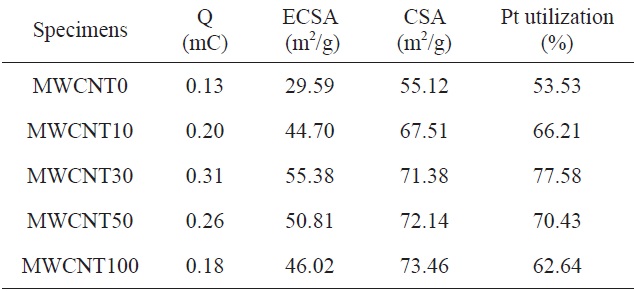
The CSA, ECSA, and Pt utilization of PtRu deposited on CBs/MWCNTs
where K is the Scherrer constant (0.89), λ is the wavelength of X radiation (0.154 for Cu Kα),
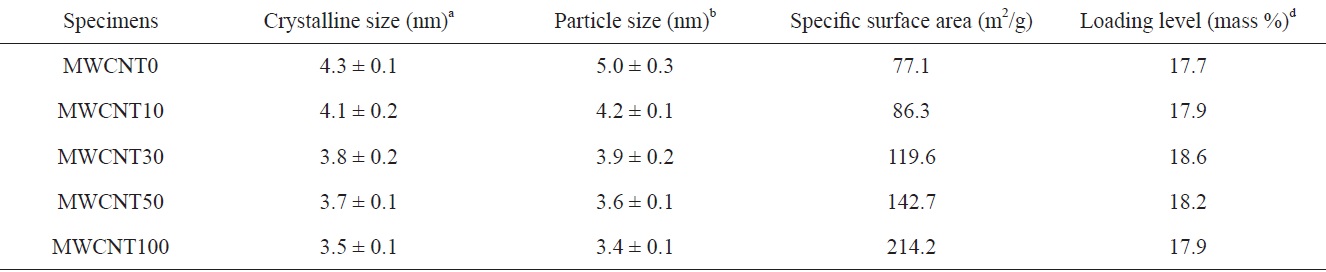
The Pt crystal size obtained from the peak of FCC (220) and the particle sizes determined from TEM results are listed in Table 1. The Pt crystal size decreased gradually from 4.3 to 3.5 nm with changing MWCNTs ratios. The PtRu particle sizes obtained from TEM images were in agreement with the results calculated from XRD. The decrease in PtRu particle sizes can be related to the increase of specific surface area by increasing the portion of MWCNTs, which have a larger specific surface area than the CBs: The specific surface area, calculated from nitrogen adsorption at 77 K using the BET method, is 77.1 m2/g for CBs and 214.2 m2/g for MWCNTs, as shown in Table 1. Consequently, the specific surface area of the binary carbon supports gradually increased with an increase in MWCNT content. This could lead to the formation of a separate secured area for the deposition of PtRu particles due to the interaction of carbon supports. Therefore, the PtRu particle dispersion improved and the particles became smaller because of the increase in the specific surface area [14].
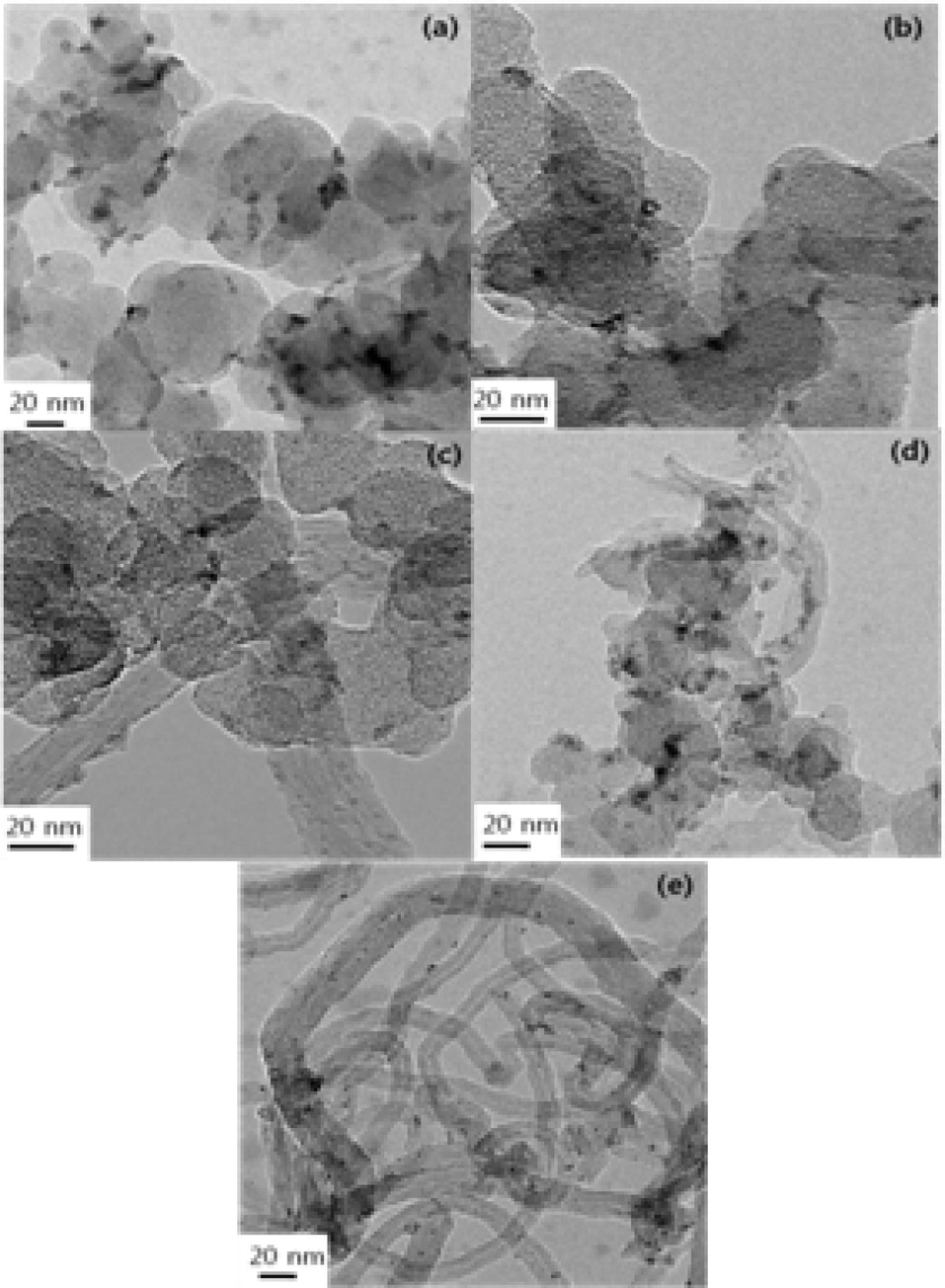
TEM images show the particle sizes and morphologies of the
catalysts on the CB/MWCNT support. As seen in Fig. 2, PtRu particles were successfully deposited on the mixed binary carbon supports regardless of mixing ratios of CBs to MWCNTs. PtRu particle dispersion improved with an increasing MWCNT content, and aggregation was not observed. It can be also seen that the PtRu particles are gradually less evident with an increasing content of MWCNTs. In particular, in MWCNT30,icles were uniformly deposited on the MWCNT30. The Pt and Ru content and loading levels of the catalysts were determined from the ICP-MS results (Table 2). The loading level of the catalysts means that the impregnated PtRu weight percent in the carbon materials is 20%, as described in the previous section. The loading level of PtRu deposits may be related to the reaction conditions of PtRu reduction and to the surface characteristics of the carbon supports. In other words, the loading level is probably dependent on the changes in surface conditions related to Pt reduction and to changes in the specific surface area owing to variations in the mixing ratios of the two carbon supports.
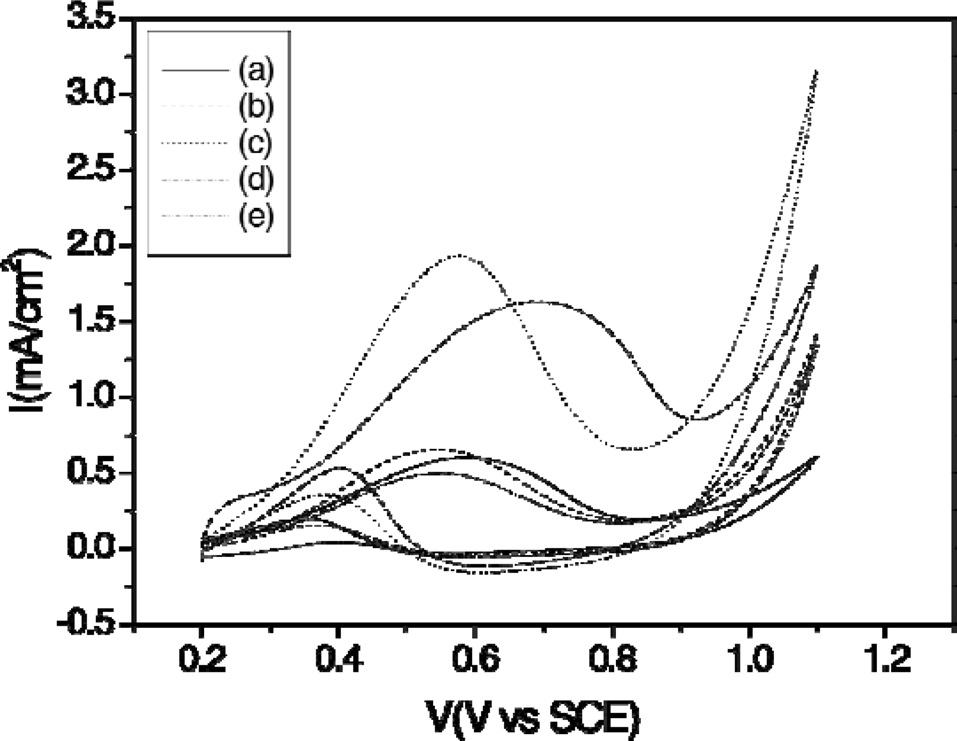
Fig. 3 shows the cyclic voltammograms (CVs) of the prepared catalysts in a 1 M H2SO4 solution. In the absence of methanol, typical surface processes for polycrystalline Pt such as hydrogen adsorption/desorption and, to a lesser extent, oxide formation and reduction can be observed in the CVs recorded on the Pt/C composite electrode. The Pt ECSA was calculated from the hydrogen desorption peak areas in the CV curves using the following formula [11].
[Table 2.] Mean size, textural properties and loading contents of the catalysts
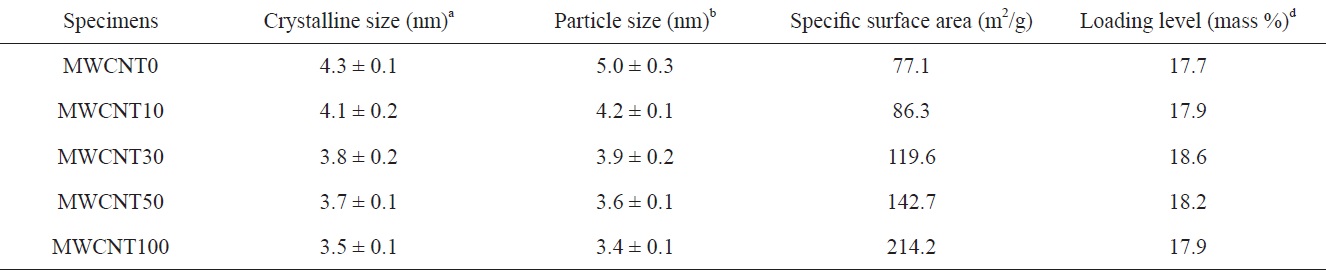
Mean size, textural properties and loading contents of the catalysts
where Q is the electric charge for hydrogen desorption and the value of the hydrogen adsorption constant for polycrystalline Pt is 0.22 mC/cm-2.
The calculated ECSA values are shown in Table 1, which also summarizes other catalyst data. The chemical surface area (CSA) of the PtRu catalysts was calculated using the following equation [11]:
where
Generally, catalysts with a smaller particle size had a higher ECSA than those with a larger particle size and distribution.
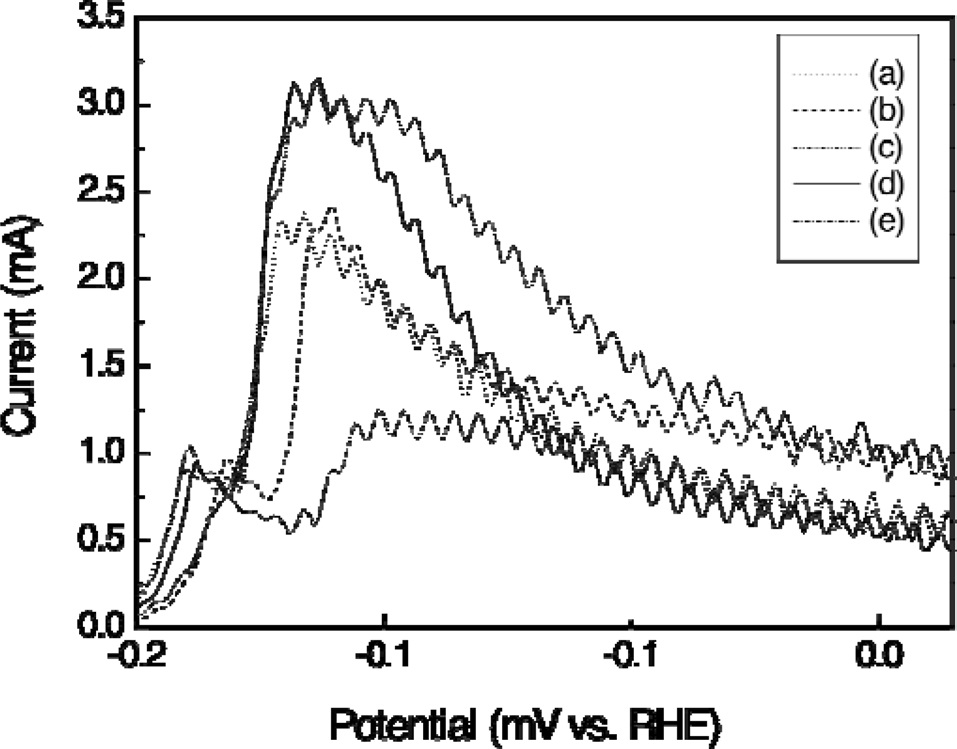
Fig. 4 shows the electrocatalytic activity of the catalysts in methanol oxidation. Significant differences in the peak current density and onset potential between the catalysts were observed, thus illustrating the beneficial role of the support materials. A comparison of catalyst CV curves shows that the peak current density of PtRu deposited on binary carbon supports was much higher than that of PtRu deposited on single supports. A definite methanol oxidation peak of the catalyst occurred at 510-690 mV
In this study, binary carbon-supported PtRu catalysts were prepared using two carbon supports,