Generally, a curing agent for epoxy matrix resins involves the use of a cross-linker such as amine and diacid anhydride. However, they have some problems, such as the toxicity of amine, combined with low heat resistance and the deterioration of mechanical properties at high temperature or at high humidity, which requires energy consumption, resulting in a long curing process [1]. Therefore, catalytic curing agents of epoxy matrix resins have been studied as substitutes for the amine or anhydride agents [2,3].
A cationic catalyst is generally used as a complex, such as BF3-ether, BF3-amine, or SbF6-epoxide. These complexes overcome the disadvantage of excessively rapid gelation, human toxicity, high hygroscopicity, and light instability [4]. Particularly, development of latent catalysts for cationic polymerization is desirable for enhancement of both the pot-life and handling of thermosetting resins [5,6]. Among them, N-benzylpyrazinium salt has been shown to be an excellent latent thermal initiator for epoxy matrix resins [7,8]. This initiator is not hygroscopic and dissolves readily in epoxy matrix resins and exhibits a longer pot-life than the more commonly used BF3-4-methoxyaniline complex [9].
Polymeric composites, such as carbon fibers/epoxy resins and carbon fibers/PEEK systems are now being used in numerous aerospace, marine, and recreational applications. The particular composites chosen depend on the application. Epoxy resins have proved to be the most versatile in this respect as the resin itself can be cross-linked with a number of different amines, anhydrides, and acids. They can also react with many other polymer substances [10-12]. The properties of these composites, however, are governed not only by the properties of reinforcing materials, such as carbon fibers, glass fibers, aramid fibers, and so on, but also by the curing agents for the matrix resins.
In this work, the curing agent, i.e., N-benzylpyrazinium hexafluoroantimonate (BPH), was used as a cationic latent thermal catalyst to improve the properties being demanded of carbon fibers-reinforced epoxy matrix composites, and to be an ecofriendly process as well. And the effect of the above catalyst on the mechanical properties of the composites was investigated by interlaminar shear strength (ILSS), critical stress intensity factor (KIC), specific fracture energy (GIC), and impact tests.
2.1. Materials and sample preparation
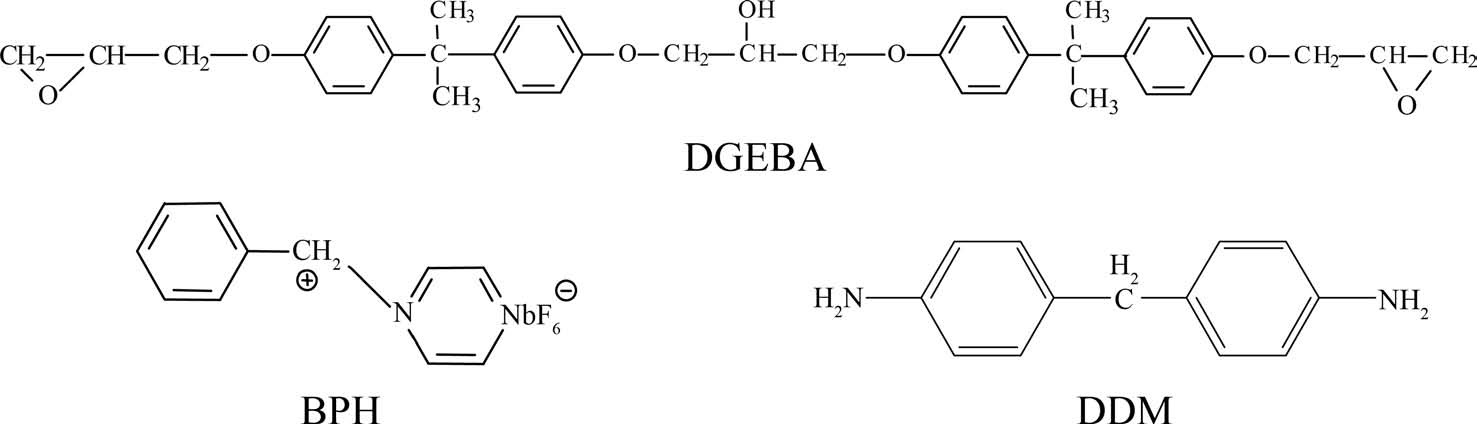
The carbon fibers used in this study were untreated and unsized PAN-based high-strength fibers, TZ-307 (12 K monofilaments), manufactured by Taekwang Co. of Korea. The average diameter of these carbon fibers was approximately 7 m, and typical tensile modulus and strength were about 245 and 3.5 GPa, respectively. Epoxy matrix resins used in this study were diglycidylether of bisphenol-A (DGEBA, YD- 128 supplied from Kukdo Chem. Co. of Korea). The cationic latent thermal catalyst, BPH, was synthesized directly [13] and diaminodiphenylmethane (DDM, supplied by Aldrich Chem. Co.) was selected as a hardener for the curing. Methyl ethyl ketone was used to reduce the high viscosity of DGEBA. Chemical structures of the BPH and DDM used as curing agents are shown in Fig. 1.
Unidirectional composite laminates were prepared by continuous impregnation of the fibers using a drum winding technique for manufacturing prepregs with subsequent hot pressing. The laminates made with 32 plies of prepregs were fabricated in a hot-press at 7.4 MPa at 150℃ for 150 min with a vacuum bagging method in a conventional composite processing [14].
To obtain an average fiber volume fraction, Vf of the composites, a small size rectangular specimen was cut from the laminate, and the side section of the specimen was polished. The number of fibers (
The average fiber volume fraction of bulk specimens was about 52% (0.2%) for all composites.
Latent properties of the BPH catalyst used were determined by the measurement of conversion as a function of curing time using the isothermal differential scanning calorimetry (DSC, Perkin Elmer Co., DSC-7) method. ILSS of the composites was conducted by a three-point short-beam bending test method to estimate the interfacial adhesion strength of the composites, according to the ASTM D 2344. The distance between supports divided by the thickness of specimens, L/d=5, and the crosshead speed was fixed at 2.0 mm/min. For a rectangular cross section of the composites, the ILSS was calculated as [15]:
where
The accuracy and range of the load cell used were 0.5% grade and 5 kN, respectively. The composites were machined along the fiber direction into 30 mm × 6 mm short-beam-shear specimens with 5 mm thickness. The distance between supports divided by the thickness of specimens, L/d=5, and the cross-head speed was fixed at 2.0 mm/min. The fracture toughness parameter, critical stress intensity factor (KIC) and specific fracture energies (GIC) of the composites may be characterized by single edge notched (SEN) test in three-point flexure. For the SEN beam fracture toughness test, the value of KIC is calculated as follows:
where
Based on the Griffith-Irwin equation [16] the resistance to crack propagation (GIC), or specific fracture energy is increased as the fracture toughness and the poisson ratio increases or as the electric modulus decreases, as follows:
where
Notches were cut using a diamond-coated saw, approximately half the depth of the specimen. The three-point bending test was conducted using an Instron Model 1125 mechanical tester according to the ASTM E-399. A span-to-depth ratio of 4:1 and cross-head speed of 1 mm/min was used. Impact strength was obtained with a Tinius Olsel Model 66 Izod Impact Tester according to ASTM method D790. The morphology of the composites was examined with scanning electron microscope (SEM, JEOL Model 840A). A small section of the non-woven mat was placed on the SEM sample holder and sputter-coated with gold (Denton Desk- 1 Sputter Coater). An Amray 3000 SEM using an accelerating voltage of 20 kV was employed to take the SEM photographs.
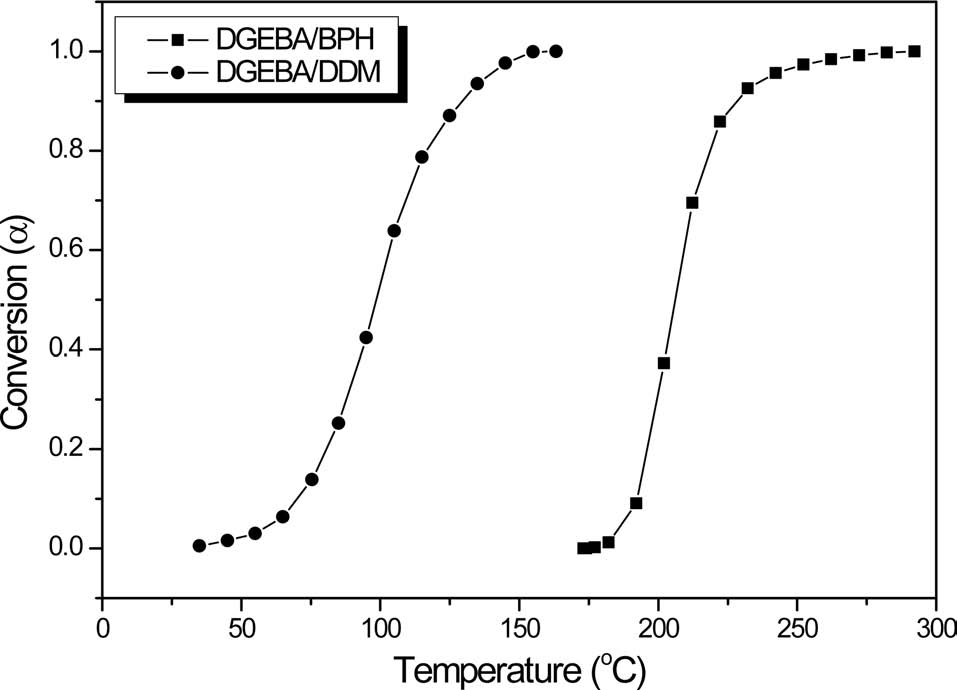
Fig. 2 shows the conversion of neat epoxy matrix resins cured by BPH and DDM as a function of curing temperature using a dynamic DSC method. The conversions of all compositions are activated at a given specific temperature. However, for the DDM curing agent, the conversion is activated at a lower temperature, resulting in a poor pot-life under room temperature condition. On the other hand, the conversion using the BPH presents a significant change in a specific temperature region as the reaction temperature increases. So, the presence of BPH used as a curing agent appears to support its possible use as a latent thermal initiator in this matrix system, in a given temperature condition.
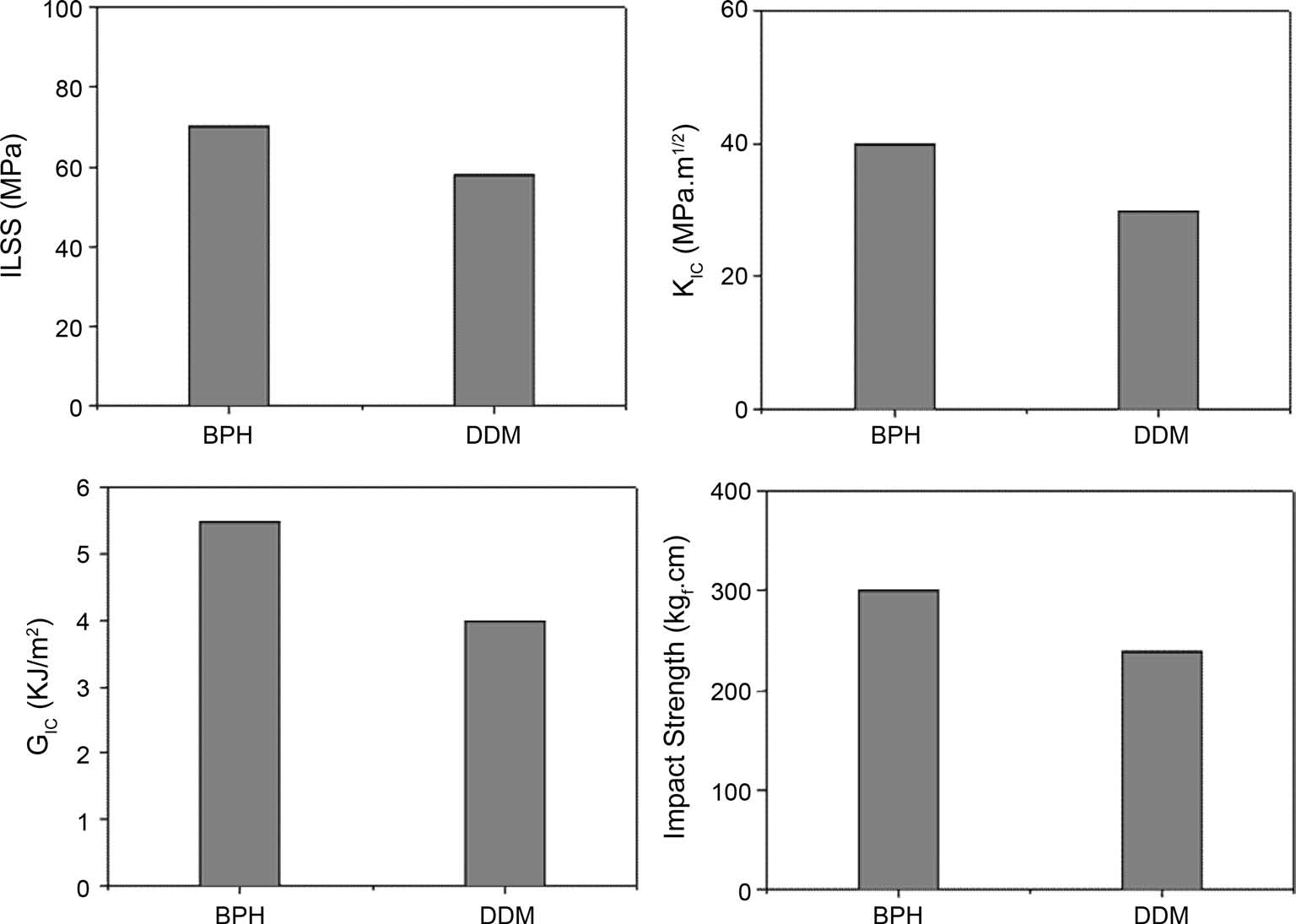
Fig. 3 shows the results of ILSS, KIC, GIC, and impact strength of the composites. The results clearly indicate that the mechanical property values in composite specimens cured by BPH are higher than those of the ones cured by DDM. As mentioned above, this can be explained as probably due to
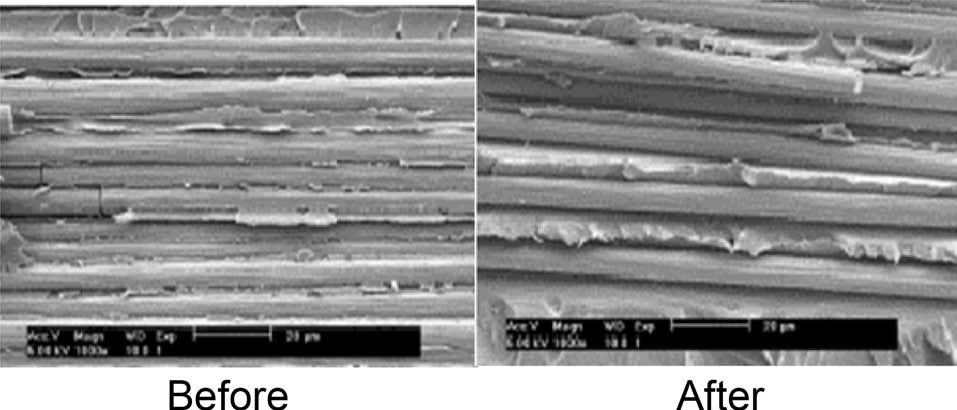
the effect of the substituted benzene group, resulting in improvement of the ductile fracture properties, leading to the increased good resistance to deformation and crack initiation of the epoxy matrix system in carbon fibers-reinforced composites [17,18]. And a good agreement can be found by SEM pictures of the fracture surfaces after impact test, as seen in Fig. 4.
Consequently, the chain segments of the BPH-cured composite system responsible for entanglements and intermolecular interactions, such as hydrogen bonding and double helical formations, mostly exist as the outer bulk side chains which is related to the free volume, including the hydroxyl groups of the epoxy
matrix system. Thus, the chain segments toughen the three-dimensional structure and consequently disperse the internal stress [19], which results in an increase of the elastic modulus in the fracture of the composite system cured by BPH.