The development of hollow carbon balls by CO2 oxidation of two types of carbon blacks was studied. Super P (SP) and Denka Black (DB) were used for this study. Specific surface area (SSA), structural parameters, and microstructures were examined using Brunauer, Emmett and Teller apparatus, X-ray diffraction spectroscopy, and transmission electron microscope (TEM), respectively. The SSAs of both oxidized carbon blacks increased after oxidation. The SSAs of raw DB and SP were 73 m2/g and 60 m2/g, respectively. Maximum SSAs of oxidized DB and SP were 152 m2/g and 253 m2/g, respectively. The d002 of DB and SP showed almost no change after oxidation. The Lc of raw DB (38A) and SP (19A) increased with increasing weight loss. The Lc of SP increased up to 254A at 96% weight loss. The SSA increased about twice in DB (148 m2/g) and about four times in SP (254 m2/g) after 3 h oxidation compared with the original carbon blacks. Through TEM observation the outer parts of the oxidized carbon blacks showed a rigid shell structure and the inner parts looked empty. Generally it looked like an angular soccer ball, so we named it ‘hollow carbon ball.’ It is expected that the hollow carbon ball can be used as catalyst supports.
Carbon black is an aggregated substance with globular particles that are used as fillers for macromolecules such as rubbers or plastics, and black pigments for inks or paints [1,2]. Since carbon black has been used for almost 100 years, the markets for carbon blacks have changed with their development [3]. The carbon black having chemical rigidity with excellent electric conductivity is used as a cathode material for secondary batteries [4,5]. It is possible to improve the characteristics of carbon black by controlling its specific surface area (SSA) and pore structure for use in many fields of application.
Oxidation reactions of carbon-based materials take place preferentially at active sites, and these oxidation reactions are affected by structural inhomogeneity [6-10]. Carbon atoms located in a less ordered area are preferentially removed, more than in an ordered area. Pores are created by such oxidation processes.
This study investigated the development of a process for producing a hollow carbon ball by the oxidation of two types of carbon blacks. SSA and structural parameter changes were examined using Brunauer, Emmett and Teller (BET) apparatus and X-ray diffraction spectroscopy (XRD), respectively. Microstructures of carbon blacks after oxidation were examined using transmission electron microscope (TEM).
Two types of conductive carbon blacks, Super P (SP, Timcal Graphite) and Denka Black (DB, Denki Kagaku Kogyo Kabushiki Kaishi) were used for this study. Physical properties of these
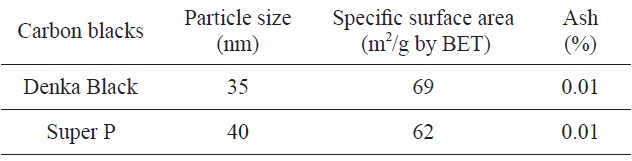
[Table 1.] Physical properties of raw carbon blacks
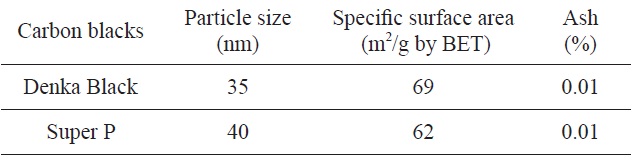
Physical properties of raw carbon blacks
carbon blacks are summarized in Table 1 which was provided by the supplying companies. As shown in Table 1, the particle sizes of SP and DB were 35 nm and 40 nm, respectively. The SSAs of raw SP and DB were 62 and 69 m2/g, respectively. Also the DB and SP should have different crystal structure, from Fig. 1. Therefore it was assumed that oxidation rate, SSA, and structural parameters would be shown to be different. The materials went through isothermal oxidation at 600℃ using a tube furnace in a carbon dioxide gas. A flow rate of CO2 gas was set to 50 mL/min and reaction times were set to 1, 2, and 3 h. The SSA data were obtained using BET apparatus at 77 K by isothermal adsorption curve of nitrogen (ASAP-2010, Micromeritics). Lattice constants and crystallite sizes of the oxidized carbon blacks were obtained
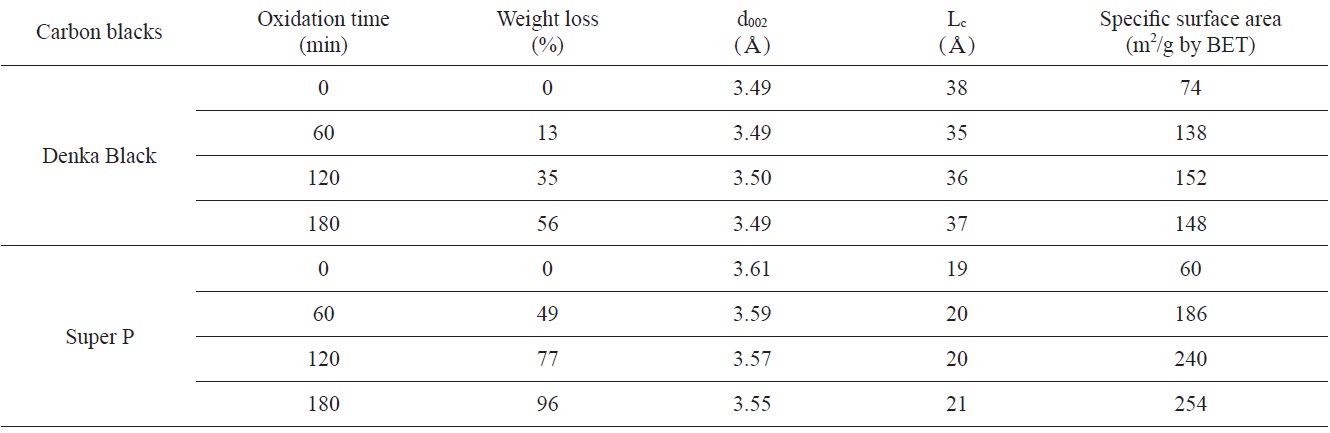
[Table 2.] Weight losses, structural parameters, and SSAs as a function of oxidation time
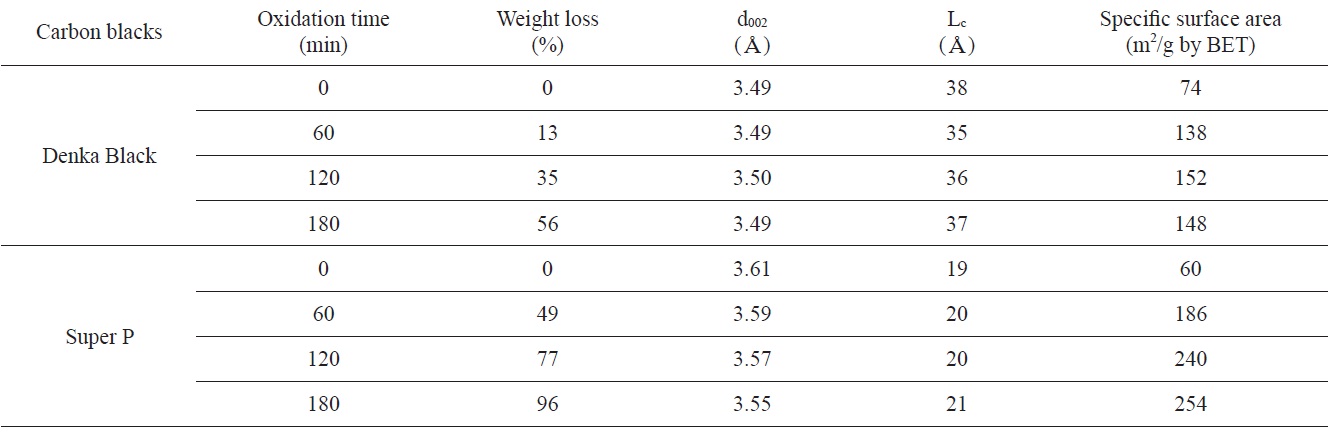
Weight losses, structural parameters, and SSAs as a function of oxidation time
from XRD (Rigaku, SWXD, X-MAX, 2000-PC). Overall sizes, shapes, and microstructures of the carbon blacks were observed using TEM (JEM2100, JEOL).
3.1. SSA and structural parameter
Weight losses, structural parameters, and SSAs with increasing oxidation time are summarized in Table 2. DB and SP were not changed significantly in their interlayer spacing after oxidation. The d002 of DB and SP were measured to be about 3.5Aand 3.6A, respectively. A crystallite size (Lc) of DB (38A) and SP (19A) increased with increasing weight loss. The Lc of SP increased up to 254A at 96% weight loss. It was considered that the increasing of Lc as the weight loss increased was caused by the preferential removal of carbon atoms located in less ordered areas. Therefore the internal structure of the oxidized carbon blacks became more ordered [7-11]. That means that the Lc of oxidized carbon blacks increase significantly with increasing weight loss.
The SSAs of original SP (60 m2/g) and DB (74 m2/g) were measured to have slightly larger values compare to the values provided by the supplying company. The SSAs of both carbon blacks were increased after oxidation. The SSA increased about twice in DB (148 m2/g) and about four times in SP (254 m2/g) after 3 h of oxidation compared to the original carbon blacks. The increasing of SSAs as the weight loss increased was caused by the removal of atoms located at less ordered areas.
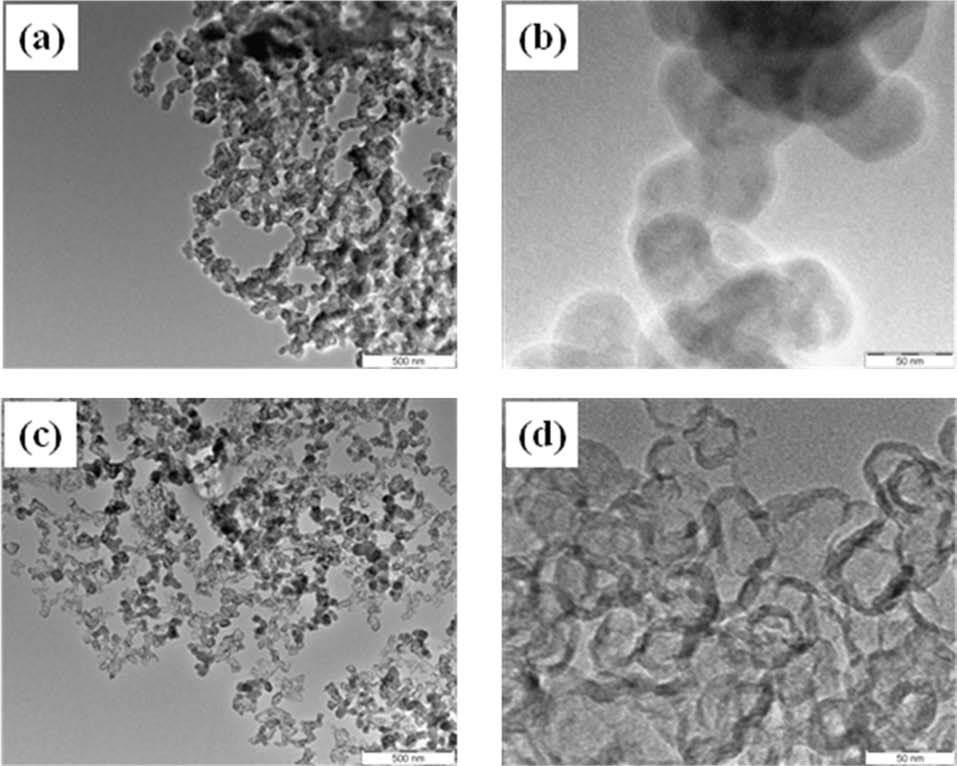
The microstructures of carbon blacks observed using TEM are shown in Figs. 2 and 3. Both carbon blacks exhibited a hollow ball shape after oxidation, where the inside was empty and the outside only had a shell-like composition. The shapes before oxidation were a round ball, while the shape was an angular ball after oxidation. The SP shows the shape of a hollow ball more clearly than DB. The particle size of the oxidized DB is smaller than the oxidized SP even though the weight loss of DB (56%) was lower than SP (77%).
Through TEM observation the outer parts of the oxidized carbon blacks showed a rigid shell and the inner parts looked empty. Generally it looked like an angular soccer ball, so we called it ‘hollow carbon ball.’ The overall thickness of the rigid shell of the oxidized DB was about 2-5 nm and the diameter of the inner empty space was about 10-20 nm. Also the overall thickness of the rigid shell of oxidized SP was about 8 nm and the diameter of the inner empty space was about 20-30 nm. It is expected that the hollow carbon ball can be used as catalyst supports.
Development of a hollow carbon ball by the oxidation of two types of carbon blacks was studied and the following conclusions can be drawn from the results.
The SSA of raw DB and SP were 73 m2/g and 60 m2/g, respectively. The maximum SSAs of oxidized DB and SP were 152 m2/g and 253 m2/g, respectively. The d002 of DB and SP showed almost no change after oxidation. The Lc of raw DB (38A) and SP (19A) were increased with increasing weight loss. The Lc of SP increased up to 254A at 96% weight loss. The SSA increased about twice in DB (148 m2/g) and about four times in SP (254 m2/g) after 3 h oxidation, compared to the original carbon blacks.
Through TEM observation the outer parts of the oxidized carbon blacks showed a rigid shell and the inner parts looked empty. Generally it looked like an angular soccer ball, so we called it ‘hollow carbon ball.’ It is expected that the hollow carbon ball can be used as catalyst supports.