In this study, polyaniline (PANI)/graphene nanosheet (GNS) composites were synthesized through chemical oxidation polymerization by changing the weight ratio of aniline monomers. To examine the morphological structure of the composites, scanning electron microscopy and transmission electron microscopy (TEM) were conducted. TEM results revealed that fibril-like PANI with a diameter of 50 nm was homogeneously coated on the surface of the GNS. The electrochemical properties of the composites were studied by cyclic voltammetry in 1 M H2SO4 electrolyte. Among the prepared samples, the PANI/GNS (having 40 wt% aniline content) showed the highest specific capacitance, 528 Fg-1, at 10 mVs-1. The improved performance was attributed to the GNS, which provides a large number of active sites and good electrical conductivity. The resulting composites are promising electrode materials for high capacitative supercapacitors.
Graphene, a carbon material with a two-dimensional nanostructure, has attracted a great deal of attention because of its high surface area, electrical conductivity, optimal mechanical stiffness, and high chemical stability [1-3]. In electrochemical measurements, multilayer graphene (from two to ten or more) is considered to be the promising electrode material for supercapacitors [4]. Conducting polymers are among the most extensively studied materials for supercapacitor electrodes, along with carbon and metal oxides. Among the conducting polymers, polyaniline (PANI) is widely used due to its low monomer cost, ease of synthesis, and environmental stability [5,6]. However, PANI has several disadvantages such as low cycle life and poor stability, because the oxidation state of emeraldine salt form of PANI can be changed during the repetitive charge/discharge process [7]. The formation of composites of PANI and graphene can potentially be used to reinforce the stability of PANI and to maximize the capacitance value by synergistically exploiting the pseudo-capacitance of PANI and the high conducting and mechanical properties of graphene [8,9].
In this work, composites of PANI/graphene nanosheet (GNS) with different weight ratios are synthesized by chemical oxidation polymerization of aniline monomer (ANI) in an aqueous dispersion of graphene. The combination of the GNS with PANI highly enhanced the electrochemical performance because the GNS serves as a supporting material and provides a large number of active sites. The electrochemical performance, morphology, and chemical structure of the prepared composites have been investigated in this study.
Graphite oxide was synthesized from natural graphite (SP-1, Bay carbon) by a modified Hummer’s method [10]. The graphite powder was added into a mixture of sulfuric acid, sodium nitrate, and potassium permanganate for acid treatment and the solution was maintained at 45℃ for 2 h. A 30% H2O2 aqueous solution was then slowly added into the solution. The oxidized and treated solution was filtered and washed with HCl (10%) and washed with water and ethanol through centrifugation (3600 rpm, 5 min) to remove residual graphite. GNS was prepared by the reduction of graphene oxide with sodium borohydride (NaBH4), as described elsewhere [11]. PANI/GNS composites were synthesized by chemical oxidation polymerization of ANI in the presence of grapheme suspension. A specified amount of GNS was added into 150 ml of 1 M HCl solution. The solution was sonicated for 2 h. Different amounts of ANI (20, 40, 60 and 80 wt%) were then added to the above solution and the solution was sonicated for 30 min. A solution of ammonium persulfate as an oxidizing agent was added dropwise into the above solution. The temperature of the solution was kept at 0-5℃ during the polymerization reaction. After 24 h, the reaction mixture was filtered and washed with distilled water and ethanol. Finally, it was dried in a vacuum oven at 60℃. All electrochemical tests were done in a three electrode system. The working electrode was prepared by casting a nafion-impregnated sample onto a glassy carbon electrode. Platinum wire and a saturated calomel electrode were then used as counter and reference electrodes, respectively. The measurements were carried out in 1 M H2SO4 electrolyte. Electrochemical measurements were performed in an Iviumstat (Ivium Technologies, The Netherlands).
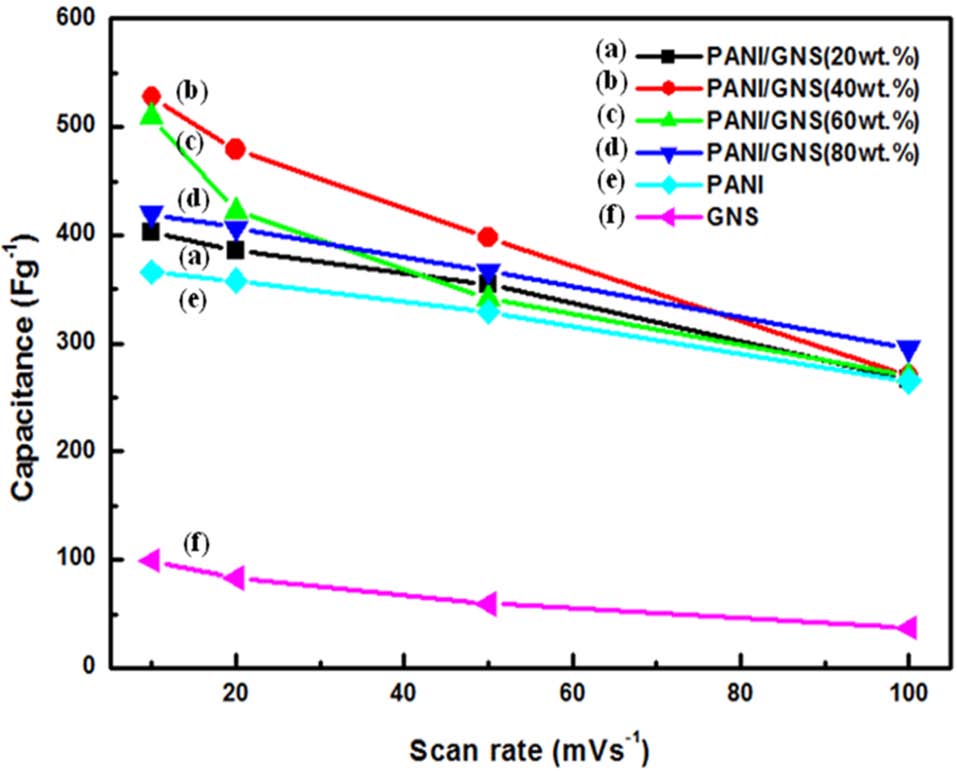
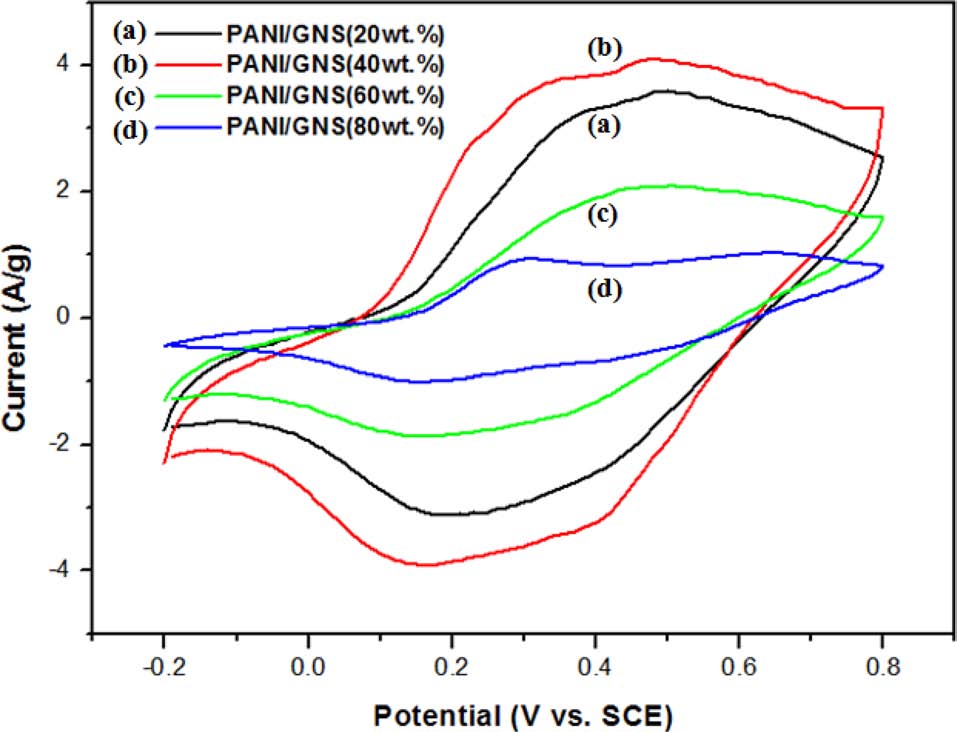
The specific capacitances of the prepared composites with different weight ratios of ANI are presented in Fig. 1. With the introduction of GNS into PANI, the PANI/GNS composites showed improved capacitance compared to that of PANI. In particular, the PANI/GNS composite having 40 wt% aniline content showed the highest capacitance among the prepared samples and it presented a maximum specific capacitance of 528 Fg-1 at 10 mVs-1. Fig. 2 shows the cyclic voltammograms of the PANI/GNS composites as a function of aniline content. They show mixed behavior of electric double layer capacitance by the GNS component and redox (or faradaic) capacitance by the PANI. These enhanced specific capacitances are due to the GNS, which provided a large number of active sites and high conductivity [12]. Beyond 60 wt% aniline content, the composite showed slightly decreased capacitance values. It is thought that aniline content over 60 wt% would produce an excessively thick coating of PANI onto GNS. It was expected that such a thick PANI coating could not endow effective surface area or a suitable pore structure for easy charge transfer and ion transport. Also, it was observed that the specific capacitance decreases as the scan rates were increased from 10 to 100 mVs-1. At a high scan rate, the diffusion of electrolyte ions was limited by structural properties and only the outer active
surface could be utilized for charge storage.
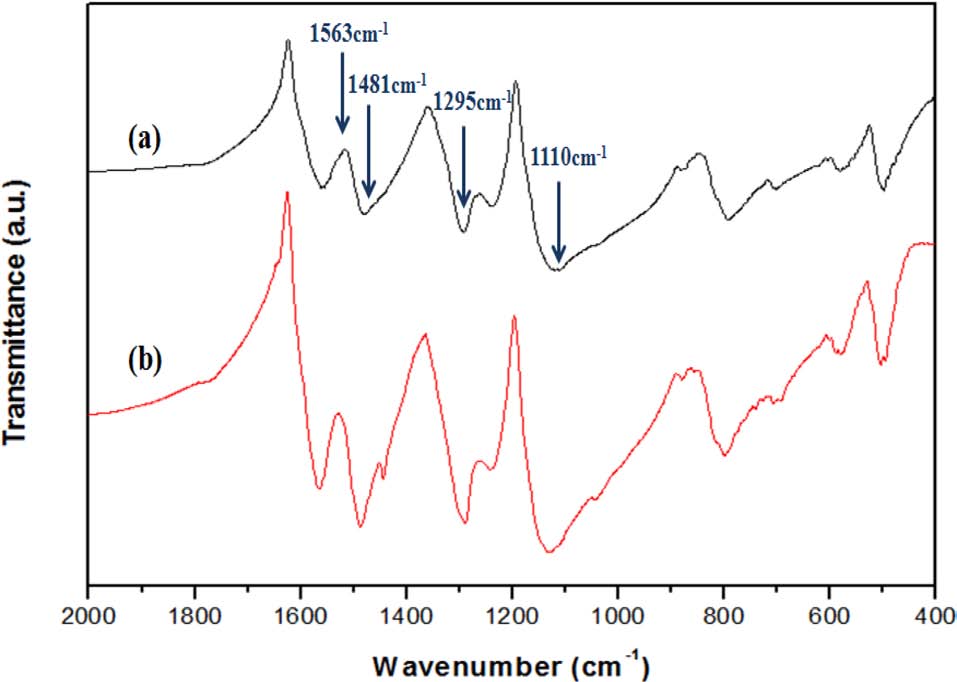
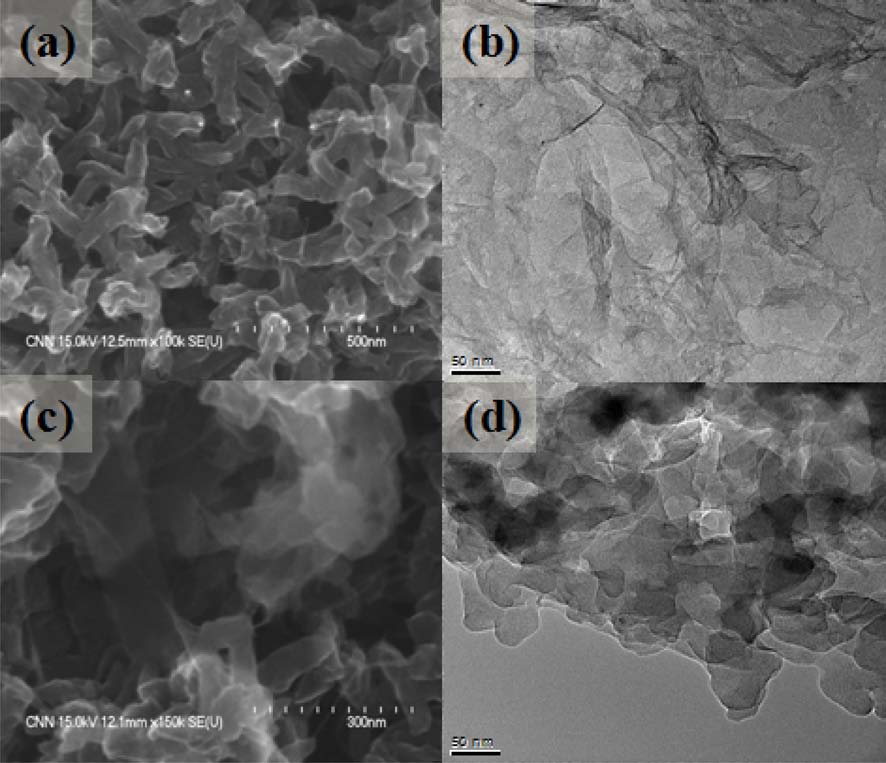
Fig. 3 shows Fourier transform infrared spectroscopy spectra of PANI nanofibers and PANI/GNS (having 40 wt% aniline content) composites. For PANI nanofibers, the absorption bands at 1563 and 1481 cm-1 are assigned to the C=C stretching of quinone rings and benzene rings. The C-N stretching vibration of the secondary aromatic amine and aromatic C-H bending appeared on the characteristic bands at 1295 and 1110 cm-1 [13,14]. Also, these bands are observed in the spectrum of PANI/GNS (having 40 wt% aniline content) composites. The absorption band at 1563 cm-1 is attributed to the skeletal vibration of the GNS, which overlapped with the C=C stretching of quinone rings of PANI [15]. The morphology and structural properties of the composites were examined by scanning electron microscopy and transmission electron microscopy images presented in Fig. 4. Transparent and wrinkled graphene sheets can be seen in Fig. 4b. In the PANI/GNS (having 40 wt% aniline content) composite, the surface of the GNS was coated by fibril-like PANI with
50 nm average diameter. Due to the electrostatic attraction between PANI and the functional groups of GNS, the PANI nanofibers were coated on the surface of the GNS [16].
In the present work, PANI/GNS composites were prepared via chemical oxidation polymerization by changing the weight ratio of ANI. The PANI/GNS composite (having 40 wt% aniline content) shows high specific capacitance of 528 Fg-1 at 10 mVs-1. The enhanced capacitance of the composite electrodes is attributed to the combined effects of PANI and GNS. The introduction of GNS into PANI increased not only the conductivity but also the number of active sites, which improved the charge transfer and ion transport throughout the electrode. The PANI/GNS composites with high specific capacitance are promising electrode materials for supercapacitors.