Dyes are synthetic aromatic water-soluble dispersible organic colorants with potential applications in various industries. The use of dyestuff materials has increased gradually due to the tremendous increase in industrialization and due to the general desire for color in everyday objects [1]. Dyes are used extensively in the paper, textile, leather, pharmaceutical, food, cosmetics, and printing industries [2,3]. The discharge of colored waste is not only damaging the aesthetic nature of the receiving streams, but also it may be toxic to the aquatic life in those streams. In addition, color interferes with the transmission of sunlight into a stream and therefore reduces the photosynthetic action [4]. Various treatment methods, such as physical, physic-chemical, biological and chemical processes have been investigated for treating dye-bearing effluents [5]. The adsorption technique has proved to be an excellent method to treat effluents, offering advantages over conventional processes, especially from an environmental point of view [6,7]. Adsorption is considered to be superior to other techniques owing to its low cost, simple design, good availability, and its ability to treat dyes in more concentrated form. Activated carbon is the most widely used adsorbent, showing great success due to its high adsorption capacity. However, its use is limited due to its high cost, and this has led to a search for cheaper substitutes. In terms of environmental protection, the utilization of these types of waste has sparked the development of processes for the production of carbon adsorbents based on plants and agricultural waste. Both the nature of the precursors and the production process strongly influence the porous structure and adsorption properties of the resulting activated carbons. The adsorption characteristics of activated carbon are determined by its pore structure (magnitude and distribution of pore volume) and surface chemistry (type and quality of surface-bound heteroatomic functional groups) [8]. Activated carbon can be prepared from a large number of sources, such as palm shells, wood, coconut shells, coal, carbon fibers and pitch [9]. Activated carbon can adsorb molecules from both liquid and gaseous phases depending on the specific surface area, pore size distribution, and surface functional groups (also denoted as surface complexes). Applications of activated carbon include drinking water purification, wastewater treatment, sweetener discoloration, food and chemical processing, solvent recovery, gasoline emission control, cigarette filters and as a treatment for industrial emission gas [10].
The aim of this research was the investigation of the methylene blue (MB) removal ability from aqueous solutions by activated carbon prepared from Sambucus nigra L. (SNL) that was chemically impregnated with KHCO3. The pH, amount of adsorbent, and time of contact of activated carbon from SNL were investigated and results were compared to Merck activated carbon and an actual sample.
2.1. Preparation of modified activated carbon
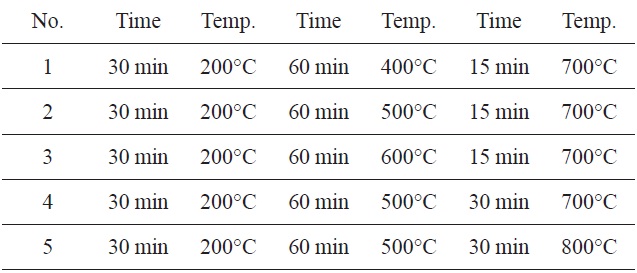
The SNL plant was used as the starting material. It was collected from the north of Iran. The stem of plant was burnt and then screened though a 120 nm sieve. The produced carbon (1 g) was chemically impregnated with KHCO3 (20%). The mixture was activated in five different furnaces (Table 1). The obtained carbon was then washed with hot distilled water. They were dried at 50-80℃ in a thermostatically controlled oven and were then filtered and analyzed using a UV spectrophotometer (Shimadzu, UV1101, Japan).
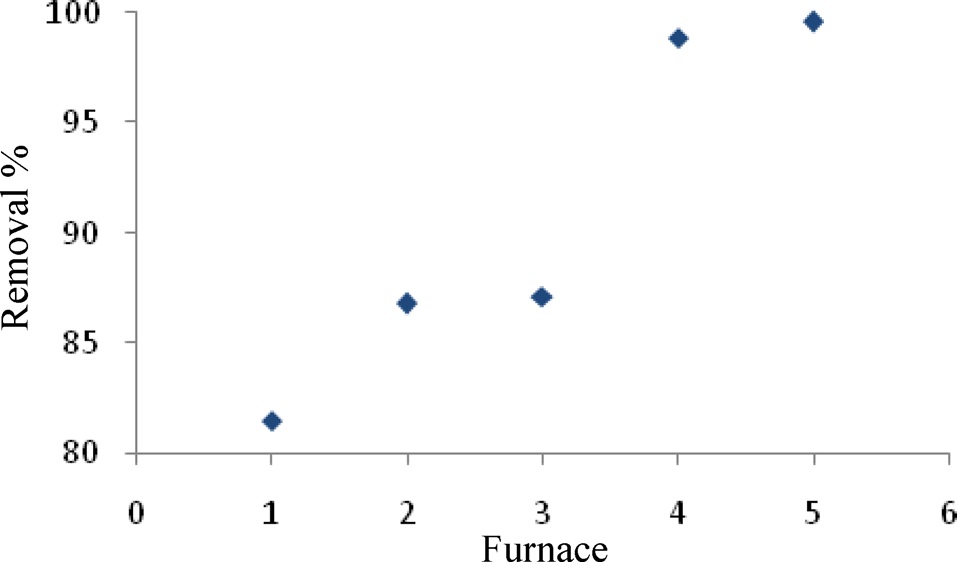
The highest adsorption capacity of MB observed in a furnace with three continuous time and temperature steps (Fig. 1) was found in No. 5. These values were 30 min at 200℃, 60 min at 500℃, 30 min at 800℃.
Therefore, the remaining adsorption experiments were carried out using these optimum times and temperatures in a furnace.
[Table 1.] Three-step preparation process at different times and temperatures
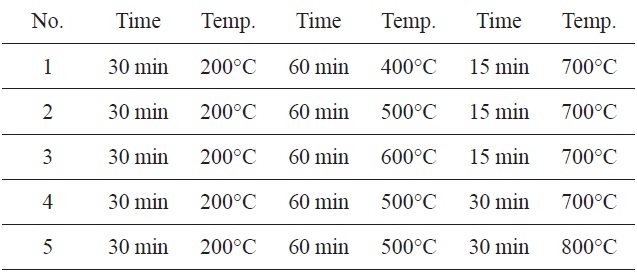
Three-step preparation process at different times and temperatures
2.2. Preparation of the adsorbate
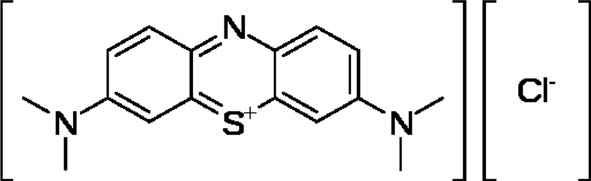
MB (Fig. 2), a basic dye used as the model adsorbate in the present study, is a monovalent cationic dye. It has a molecular formula of C16H18N3SCl and a molecular weight of 319.86 (g/ mol). It was used as an adsorbate without any purification. A stock solution of 1000 mg/L was prepared by dissolving an appropriate quantity of MB. The working solutions were prepared by diluting the stock solution with distilled water to procure the appropriate concentration of the working solutions. The chemical structure of MB is shown in Fig. 2. The concentration of MB was determined using a UV-Vis spectrophotometer at a wavelength of 620 nm.
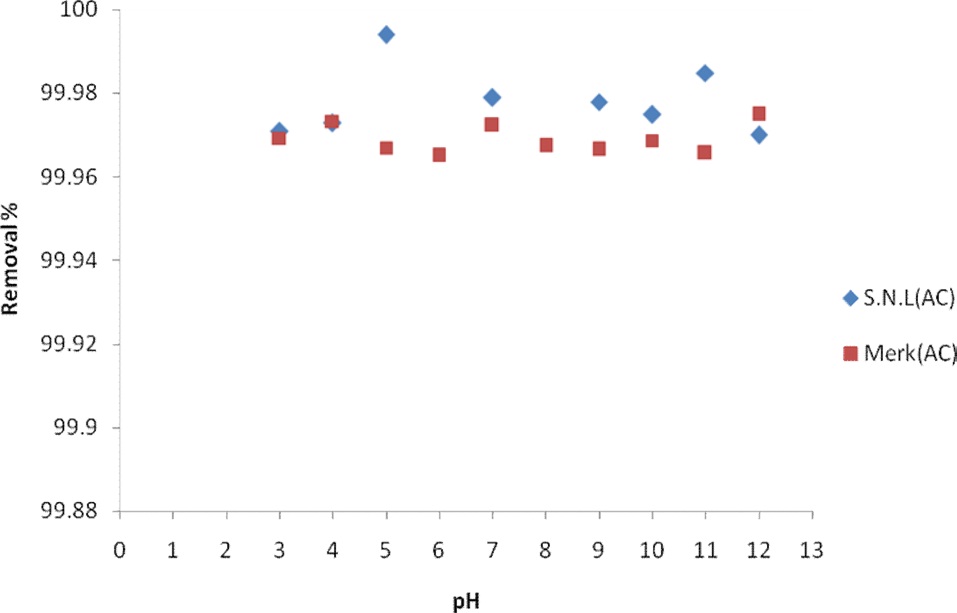
The pH of the solution from which adsorption occurs may influence the extent of adsorption. The pH affects adsorption in that it governs the degree of ionization of the acidic and basic compounds. In general, the initial pH value may enhance or depress the uptake. This is attributed to the change in the charge of the adsorbent surface with the change in the pH value [8]. In this work, the effect of the pH on the adsorption of MB by activated carbon derived from SNL was studied over a pH range of 3 to 12. In the experiments, 0.035 g of activated carbon was added to 50 mL volumes of MB aqueous solution having an initial concentration 25.35 mg/L for a constant sorption time of 10 min. The adsorption behavior of the activated carbon of SNL was studied by
Here,
[Table 2.] Characteristics of the activated carbon derived from SNL

Characteristics of the activated carbon derived from SNL
2.4. Effect of adsorbent dosage
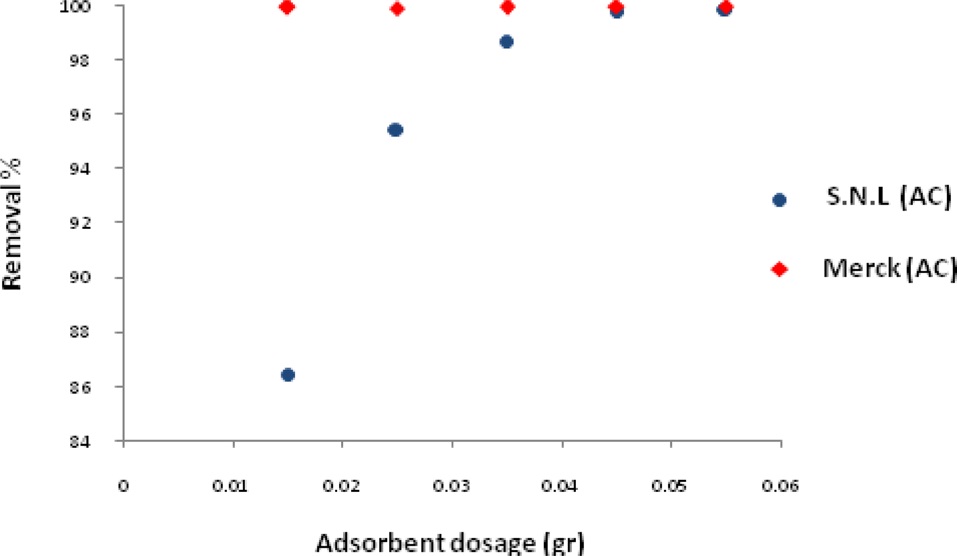
The effect of activated carbon derived from the SNL dosage on the adsorption process was investigated varying the adsorbent dosage from 0.015 g/50 mL-0.055 g/50 mL in the MB solutions. The mixture was agitated in a shaker for 10 min and the samples were then filtered and centrifuged. They were subsequently analyzed for the remaining MB concentration at a wavelength 620 nm.
2.5. Characterization of the activated carbon after the preparation of modified activated carbon
The Brunauer-Emmett-Teller (BET) method was used to calculate the surface area. The total pore volume (vtotal) and the average pore diameter (dav) were derived from the Barrett-Joyner- Halenda (BJH) method.
The BET surface area of activated carbon fiber derived from SNL as used in this paper was 426.9 (m2/g) with a total pore volume of 0.126 (cc/g). The average pore diameter of SNL was found to be 1.186 nm. Table 2 shows the characteristics of the activated carbon derived from SNL.
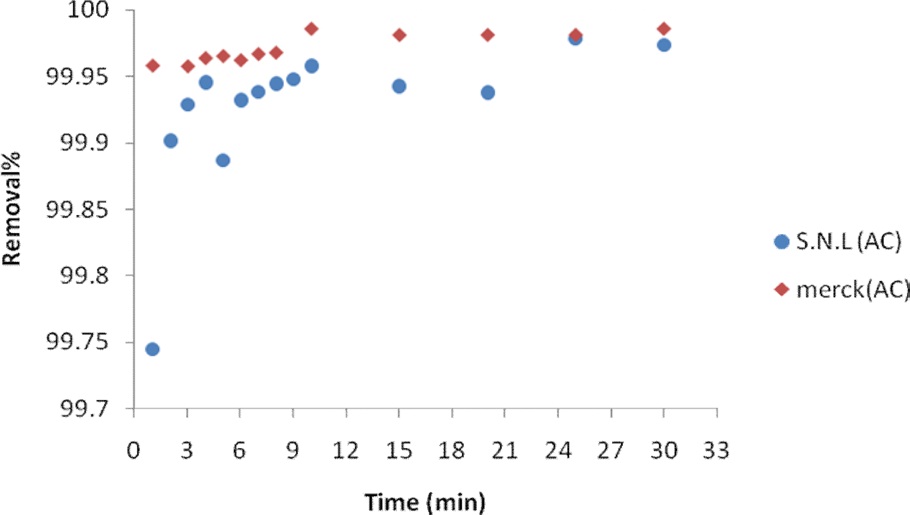
The effect of the contact time on the adsorption of MB on the activated carbons from SNL and Merck was studied. A 0.035 g sample of activated carbon derived from SNL was added to 50 mL of MB solution and agitated in a shaker in 1-30 min. The experiments were carried out at an initial MB concentration of 25.35 ppm. The samples were withdrawn at a suitable time interval, filtered, and centrifuged and the filtrate was analyzed for the remaining MB concentration spectrophotometrically at 620 nm.
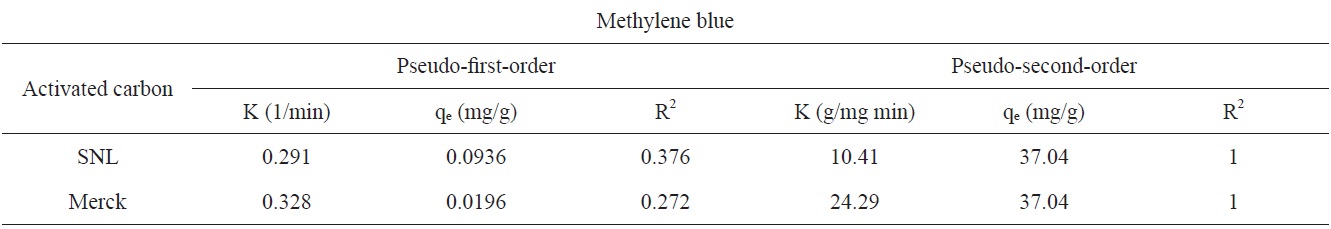
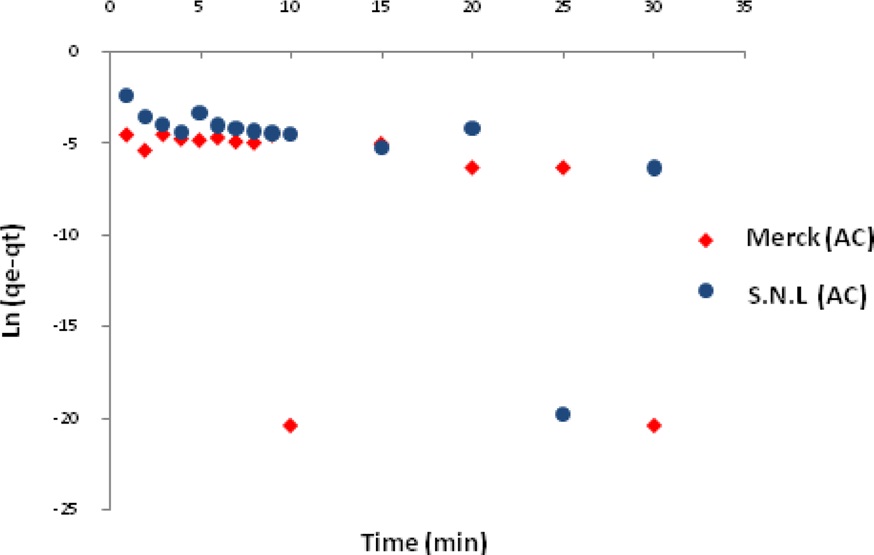
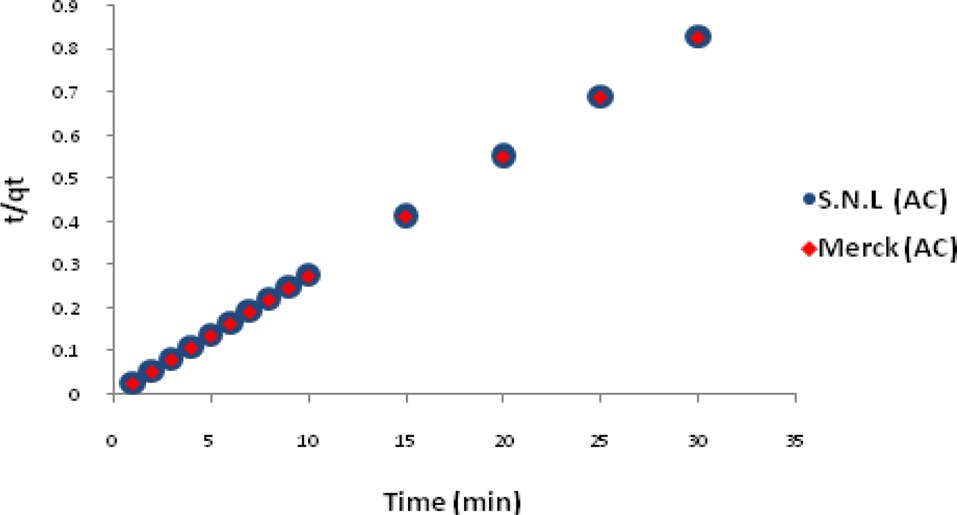
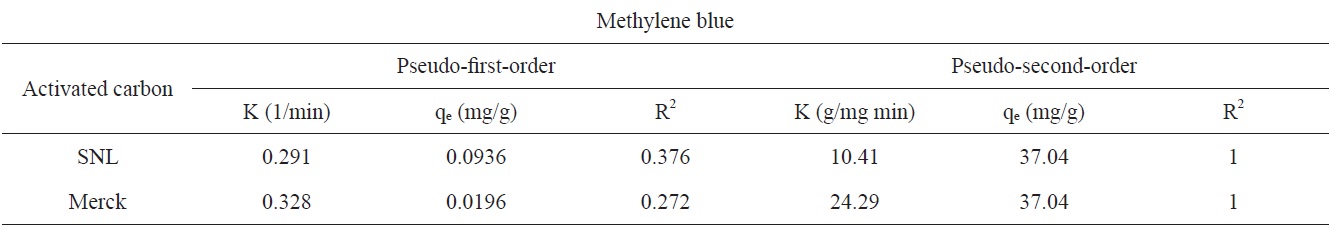
The adsorption kinetics of MB on activated carbon derived from SNL can be studied by applying a pseudo-first-order and pseudo-second-order rate equation of the types typically used to determine the adsorption of an adsorbate from an aqueous solution. These are expressed below.
In these equations, qe and qt are the amount of dye adsorbed per unit mass of the adsorbent (mg/g) at the equilibrium time and at time t, respectively, and kad is the rate constant [11].
0.035 g of activated carbon derived from SNL was added to 50 mL of MB solution (25.35 ppm) and the mixture was agitated in a shaker for different times, after which it was filtered. The remaining MB concentration filtrates were analyzed with a spectrophotometer at 620 nm. This experiment was also carried out using Merck activated carbon.
Equilibrium adsorption isotherm studies are required in the screening of a sorbent for potential use as a form of wastewater treatment. This process can help to evaluate the affinity of a sorbent for a particular sorbate [12].
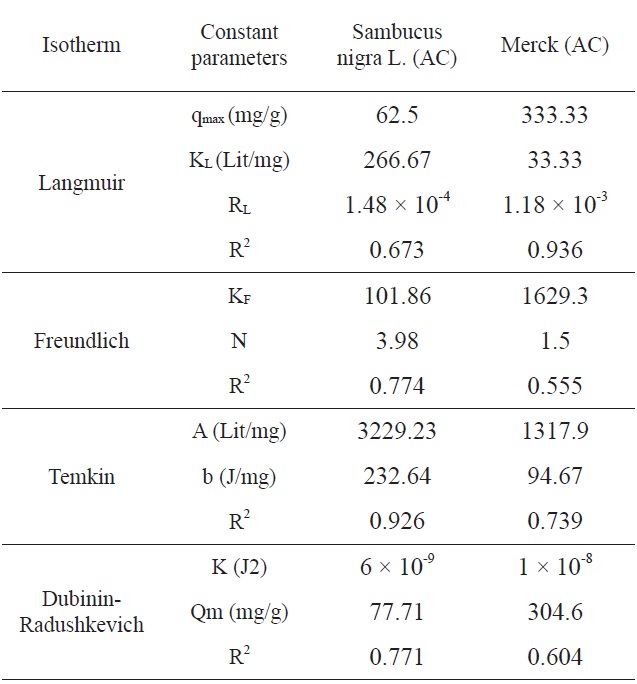
The experimental data results were analyzed by means of Langmuir, Freundlich, Temkin and Dubinin-Radushkevich (DR) isotherm equations. The Langmuir equation can be applied for the monolayer sorption onto a surface of a finite number of identical sites. The linearized form of the Langmuir isotherm is presented by the following equation [13]:
Here, ce is the equilibrium concentration (mg/L), qe is the adsorption capacity at equilibrium (mg/g), kL denotes the Langmuir adsorption constant (L/mg) and qmax is the theoretical maximum adsorption capacity (mg/g) [14].
The sorption of MB was carried out at different initial MB concentrations ranging from 8.45 to 59.15 ppm; optimum conditions of all pertinent factors were used.
The essential characteristics of the Langmuir isotherm can be expressed in terms of the dimensionless constant separation factor RL, as determined by the following equation:
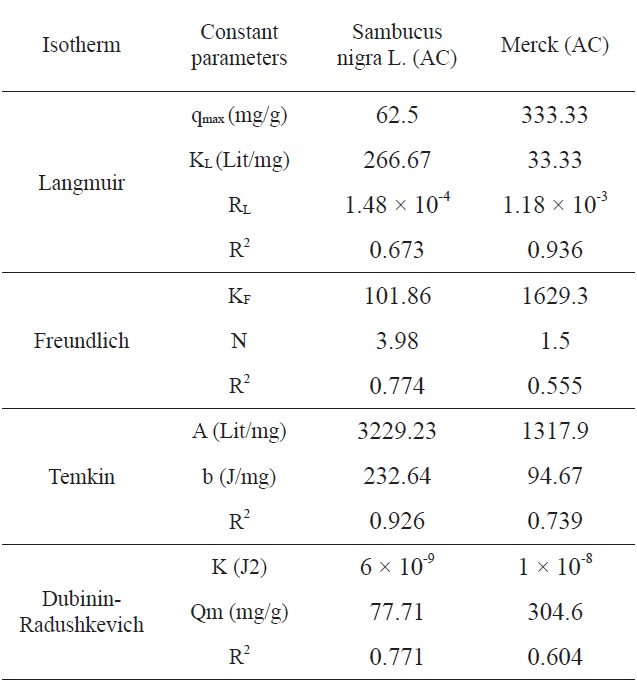
[Table 3.] Comparison of the coefficients isotherm parameters by MB adsorption onto SNL and Merck AC
Comparison of the coefficients isotherm parameters by MB adsorption onto SNL and Merck AC
Here, C0 is the highest initial concentration of the adsorbate (mg/L) and kL is the Langmuir constant (L/mg). The value of RL indicates the shape of the isotherm?whether it is unfavorable (RL > 1), linear (RL = 1), favorable (0 < RL < 1) or irreversible (RL = 0). RL values between 0 and 1 indicate favorable adsorption.
The Freundlich isotherm is derived by assuming a heterogeneous surface with a non-uniform distribution of heat of adsorption over the surface.
In this equation, KF is the adsorption capacity at the unit concentration and 1/n is the adsorption intensity. 1/n values indicate the type of isotherm ? whether it is irreversible (1/n = 0), favorable (0 < 1/n < 1), or unfavorable (1/n > 1). Eq. (6) can be rearranged into a linear form [15]:
The linear form of the D-R isotherm equation can be expressed as [16],
where Qm is the theoretical monolayer saturation capacity (mg/g), K is the D-R model constant (mg2/J2), and ε is the Polanyi potential. The latter value is expressed as follows:
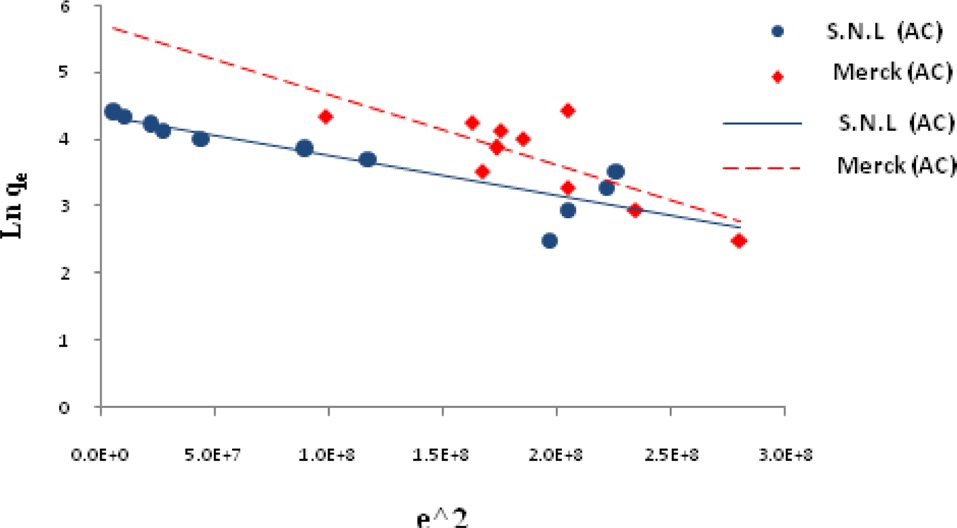
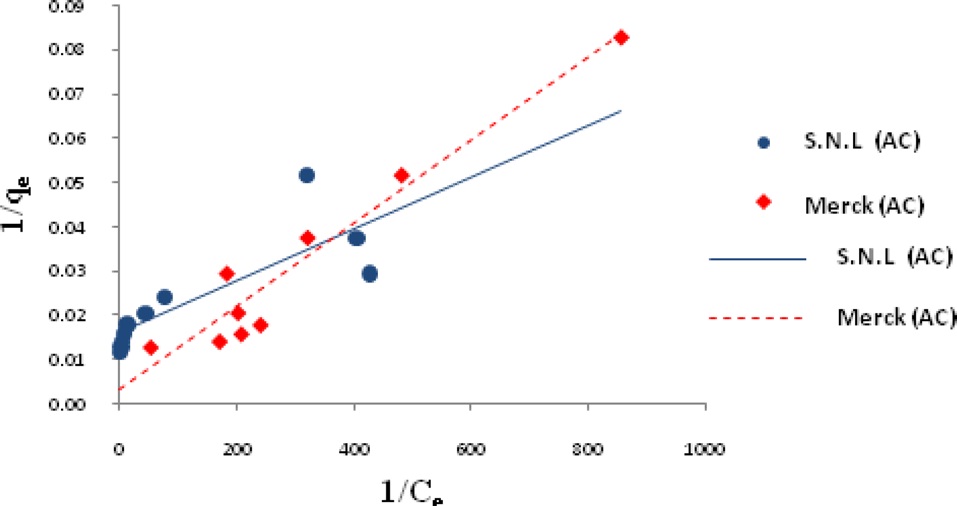
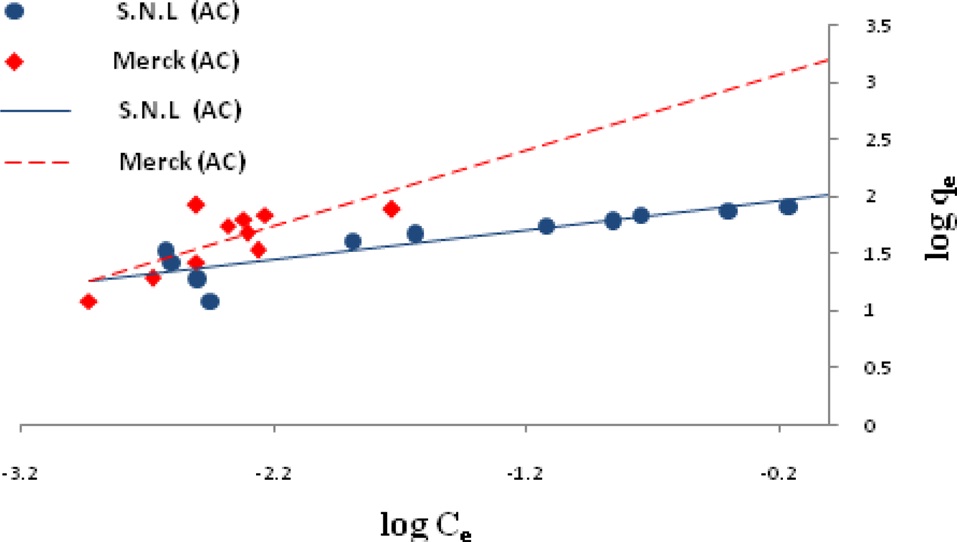
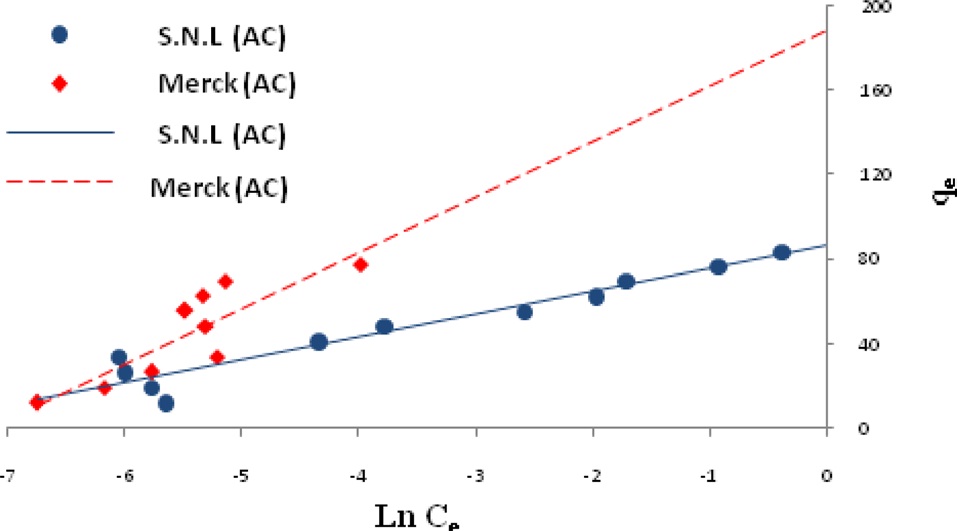
Ln qe vs.ε2 was plotted at different temperatures for both the SNL and Merck activated carbon, as shown in Fig. 3. A list of the parameters obtained together with the R2 values are given in Table 3.
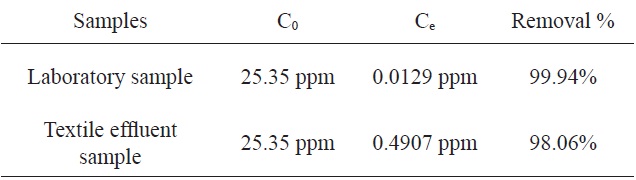
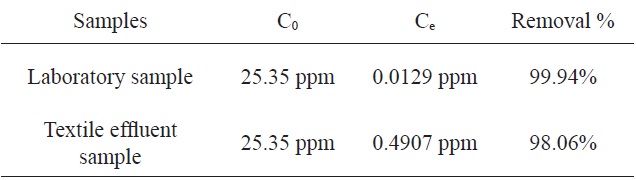
The adsorption of MB by activated carbon from SNL with KHCO3 was investigated using a textile factory effluent sample and the results were compared to those from a laboratory sample. Table 4 shows the comparison of the percentage adsorption of MB with the two samples. According to this table, the percentages of adsorption of MB using the activated carbon from SNL were identical for the two samples.
[Table 4.] Comparison of the percentages of adsorption of MB by the two samples
Comparison of the percentages of adsorption of MB by the two samples
Fig. 4 shows the effect of the pH on the removal percentage of MB dye for the activated carbon derived from SNL and from Merck activated carbon. The sorption at pHs ranging from 3 to 11 was remarkable, and there was no significant difference in the dye concentration remaining when the pH was increased because the solution pH was higher than the pHzpc value [17].
3.2. Effect of the adsorbent dosage
Fig. 5 shows the effect of the adsorbent dosage on the adsorption of MB for activated carbon derived from SNL and for Merck activated carbon. It was observed that the removal of MB increased to 0.045 and then remained nearly constant. The increase in the color removal percentage was due to the increase in the size of the available sorption surface.
3.3. Effect of the contact time
Equilibrium levels were mostly achieved in 10 min. A slight increase occurred during the next 10 min. Fig. 6 shows the effect of the contact time on the adsorption of MB for activated carbon derived from SNL and for the Merck activated carbon.
Figs. 7 and 8 show the pseudo-first-order and pseudo-secondorder plots, respectively. As shown, the pseudo-second-order plot gives a straight line with a correlation coefficient R2 of 1 for SNL (activated carbon) and 1 for Merck (activated carbon), indicating that the applicability of the pseudo-second-order equation was better than that of the pseudo-first-order plot, with R2 = 0.376 for SNL (activated carbon) and R2 = 0.272 for Merck (activated carbon) only. Table 5 shows the kinetics constants for the pseudo-first and pseudo-second-order models.
According to the R2 values, the fitting of the Temkin isotherm to the experimental data is better than that of the other isotherms
[Table 5.] Kinetics constants for pseudo-first and pseudo-second-order models
Kinetics constants for pseudo-first and pseudo-second-order models
in this study for SNL. In addition, the fitting of the Langmuir isotherm to the experimental data is better than that noted with
the other isotherms in this study for the Merck activated carbon. Figs. 3,9-11 show the Langmuir, Freundlich, Temkin and D-R models, respectively, for the adsorption of MB by SNL and Merck activated carbon.
This study investigated the adsorption mechanism of MB (a cationic dye) on activated carbon samples from an aqueous solution as a function of the pH, amount of adsorbent, contact time, adsorption kinetics and adsorption isotherm. The results were compared to those using Merck activated carbon and a real sample. According to the results, prepared activated carbon can be used as a low-cost adsorbent comparable to commercial forms of activated carbon for the removal of textile dyes from textile wastewater processes. The cost is low because SNL is a weed in the north of Iran. The highest adsorption capacities were observed in three continuous steps at the times and temperatures of 30 min at 200℃, 60 min at 500℃, 30 min at 800℃. According to the results, the pH did not have a significant effect on the color removal activity. The highest adsorption capacity was obtained when 0.035 g of adsorbent. The adsorption equilibrium for color removal was reached within 10 min. The isotherm data was well fitted to the Temkin model, and the sorption process obeyed the pseudo-second-order kinetic model.