



Carbon nanotubes (CNTs) are increasingly attracting scientific and industrial interest because of their outstanding characteristics, such as a high Young’s modulus and tensile strength, low density, and excellent electrical and thermal properties. The incorporation of CNTs into polymer matrices greatly improves the electrical, thermal, and mechanical properties of the materials. Surface modification of CNTs can improve their processibility and dispersion within the composites. This paper aims to review the surface modification of CNTs, processing technologies, and mechanical and electrical properties of CNT-based epoxy composites.
Epoxy resins are widely used in practical applications, such as coatings, electronics, adhesives, and as matrices for composites because of their excellent mechanical properties, high adhesiveness to many substrates, and good heat and chemical resistances. However, the materials are inherently brittle as a result of their high crosslinking density, which puts a constraint on many engineering applications. Many attempts have been made to improve their physical properties using liquid elastomers, thermoplastics, and inorganic particles [1-4].
Recently, epoxy composites containing carbon nanotubes (CNTs) have received a tremendous amount of attention. CNTs can be constructed with length-to-diameter ratios that are significantly higher than those of any other materials, providing them with extraordinary mechanical, electronic, and thermal properties [5,6]. Thus, CNTs have tremendous potential in many scientific and technological applications. In particular, they would be useful as a filler in epoxy composites for improving the performance of the resulting composites [7-9].
However, the low solubility and weak dispersibility of CNTs in common solvents and epoxy matrices have limited their application in this area. Methods that have proven effective for improving the dispersion of CNTs can be divided into the categories of mechanical, physical, and chemical. Ultrasonic dispersing and high-shear mixing are examples of commonly used mechanical methods. Physical methods involve the adsorption and/or wrapping of polymers or surfactants to the surface of the CNTs, and chemical methods consist of covalent chemical bonding (grafting) of polymer chains to the CNT surfaces, which dramatically improves interfacial interactions between them and the epoxy matrix [10-15].
In this paper, the surface modification of CNTs and methods used to process them are reviewed in detail. In addition, the mechanical and electrical properties of CNT-based epoxy composites are discussed.
2. Surface Modification of CNTs
It is difficult to disperse pristine CNTs (P-CNTs) in a polar epoxy matrix because of their large surface area and strong intrinsic van der Waals forces, which cause them to aggregate. The dispersion of CNTs in epoxy resins is one of the most important challenges that needs to be overcome in order to fully realize their potential in epoxy-based CNT composites. Therefore, development of processing techniques that effectively reduce the aggregation of nanotubes within an epoxy resin is vital [16,17].
Many different chemical modification techniques have been studied for enhancing interfacial adhesion between CNTs and an epoxy matrix. Both covalent and non-covalent methods have been investigated for functionalizing the CNT surfaces. Covalent surface modification involves the formation of a chemical linkage between the polymer chains of the epoxy resins and functional groups on the surface of the CNTs. Non-covalent functionalization methods, which include solution mixing, melt mixing, and in situ polymerization, enable the conjugated structure of CNT sidewalls to be retained: however, the interfacial interaction between the nanotubes and the epoxy matrix are generally poor. For both of these approaches, it is necessary that the sidewalls be modified without significantly altering the desirable properties of the CNTs [18-21].
Acid oxidation is a well-known method for introducing reactive oxygen-containing moieties, such as carboxyl, carbonyl, and hydroxyl groups, onto CNT surfaces. This method uses strong acids such as HNO3 and H2SO4. Oxidized CNTs have demonstrated improved dispersion and interfacial behavior within various polymer matrices [22,23].
Sidewall functionalization of CNTs with organic chains or functional groups is another effective way to improve the dispersion and reinforce the combination of CNTs with the epoxy matrix. Reactive oxygen-containing moieties produced by acid oxidation can be further transformed into other functional groups using acryl chloridization, amination, esterification, and a variety of other methods [24,25].
Attachment of functional groups and polymer chains to CNTs cannot only improve dispersion of CNTs in the epoxy matrix but also enhance the binding strength at the polymer-CNT interface. Coupling between CNTs and the polymer matrix is also extremely important for efficient transfer of external stress to the nanotube structures [26,27].
2.1. Oxidation of CNTs (O-CNTs)
2.1.1. Oxidation by a mixture of H2SO4/HNO3
A general procedure for oxidizing P-CNTs using a mixture of H2SO4/HNO3 is as follows. P-CNTs are placed in an oven at 100℃ for 2 h. The oxidation of CNTs is carried out in a threenecked round-bottomed flask equipped with a reflux condenser, mechanical stirrer, and thermometer. P-CNTs (0.2 g) are immersed in a mixture of concentrated H2SO4/HNO3 (3:1 v/v) to remove impurities from the surface of the nanotubes. The mixture is then sonicated in a water bath at 40℃ for 1-3 h, filtered, and the remaining solid is washed by repeated rinsing with deionized water until the pH reached 6-7. Finally, the acid-treated CNTs are dried at 100℃ for 24 h in a vacuum oven. This procedure
[Table 1.] Elemental composition of CNTs before and after acid treatment [17]
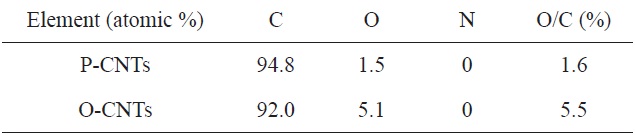
Elemental composition of CNTs before and after acid treatment [17]
results in the formation of carboxyl groups on the surface of the CNTs (abbreviated as O-CNTs) [17,28].
Jin et al. [17] demonstrated the oxidation of P-CNTs with a 3:1 mixture of concentrated H2SO4 and HNO3 by stirring at 40℃ for 4 h. Table 1 shows the elemental composition of CNTs before and after acid treatment. The O/C ratios of P-CNTs and O-CNTs samples were 1.6% and 5.5%, respectively, which confirmed the effectiveness of the acid treatment process in generating carboxyl groups on the CNT surfaces. They used Raman spectroscopy probe the structural alterations of the CNTs, and their results are shown in Fig. 1. The intensity ratio of the D and G bands (ID/IG) of the CNTs increased from 1.56 to 1.71 with acid treatment, as a result of the formation of sp3-hybridized carbon defect sites that resulted from the creation of functional groups. Similar results were observed by Hsu et al. [16] and Hadjiev et al. [28] using multi-walled CNTs (MWCNTs).
2.1.2. Oxidation by a mixture of H2SO4/H2O2
A general procedure for oxidizing P-CNTs using a mixture of H2SO4/H2O2 is as follows. P-CNTs are oxidized by a mixture of H2SO4/H2O2 (3:1 v/v) in a round-bottomed flask at 40℃ for 4 h. The resulting dispersion is diluted in deionized water to remove the residual acid and then filtered. O-CNTs are then obtained after drying in a vacuum at 60℃ for 12 h [29].
Luo et al. [29] studied the oxidation of P-MWCNTs using a mixture of H2SO4/H2O2. Their Raman spectroscopy results indicated that the
[Table 2.] Raman spectroscopy of untreated and treated MWCNTs [30]
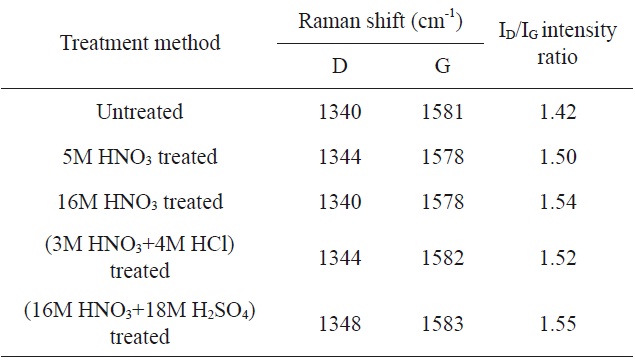
Raman spectroscopy of untreated and treated MWCNTs [30]
2.1.3. Oxidation by HNO3
A general procedure for oxidizing P-CNTs using HNO3 is as follows. Dried P-CNTs are stirred into a HNO3 solution (1:3 w/w) and the mixture is boiled at 100℃ for 1-2 h with stirring at 300 rpm. To eliminate the HNO3, the mixture is washed repeatedly with deionized water until the pH approaches 6-7, and then the modified CNTs are dried in an oven at 100℃ [30].
Kim et al. [30] demonstrated the oxidation of MWCNTs using HNO3. The Raman shift values and the
2.1.4. UV/O3-treated CNTs (UV/O3?CNTs)
A general procedure for oxidizing P-CNTs using O3 is as follows. P-CNTs (1 g) are placed in a homemade vertical reactor. O3 (5 wt% in O2) is continuously passed through the reaction chamber at room temperature during the oxidation process. The gas flow rate and humidity inside the reactor are kept at 150 L/h and 2%, respectively, and the reaction time varies from 0.5 to 6 h. For H2O-assisted ozonolysis, H2O and O3 are introduced into the reaction chamber simultaneously. The humidity in the chamber and the flow rate of the mixture are kept at approximately 60% and 150 L/h, respectively [31].
Peng et al. [31] described a method for CNT oxidation at room temperature using O3 with added H2O vapor. The resulting CNTs were characterized using Fourier-transform infrared spectroscopy (FT-IR), X-ray photoelectron spectroscopy (XPS), scanning electron microscope spectroscopy (SEM), and transmission electron microscopy (TEM). As shown in Table 3, the O/C atom ratio obtained from the XPS increased from 0.01 (P-MWCNTs) to 0.07 for O-MWCNTs and 0.14 for H2O-O-MWCNTs.
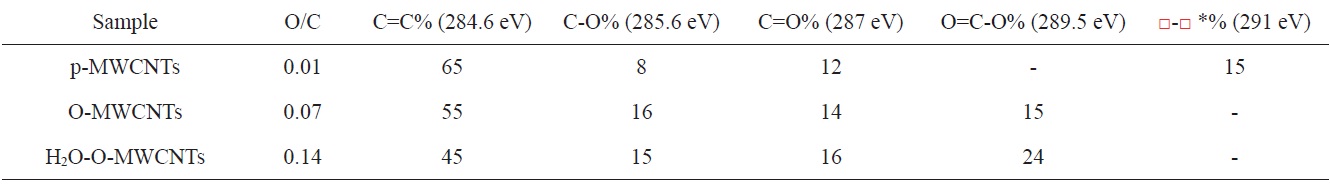
Chemical composition of the functional groups on surface of P-MWCNTs, O-MWCNTs and H2O-A-MWCNTs [31]
In the presence of H2O vapor, the O3 oxidant might partially decompose to generate hydroxyl radicals. TEM imaging revealed damaged graphite sheets as well as formed amorphous carbon entities, implying that the ozonization initiated at the outer layer and subsequently progressed to the inner layer of the MWCNTs.
2.2. Chemical treatment of CNTs
Enhancement of the interaction of CNTs with an epoxy matrix can be achieved by covalent attachment of functional groups to the nanotube walls that can react directly with the epoxy chains. The key issue in obtaining a successful covalent functionalization is the selection of the correct organic molecule, which provides both efficient grafting to the CNT surface and reactivity towards the epoxy matrix. The construction of a CNT-epoxy matrix covalent bond constitutes the strongest type of interfacial interaction and is superior to physical interactions such as van der Waals forces [17,22,29,32].
2.2.1. Amine functionalization of CNTs (amino-CNTs)
Amino-CNTs have been developed in recent years for improving the dispersion and interfacial adhesion of CNTs within epoxy resins. The amino-CNTs were obtained by direct coupling between an organic amine and carboxylic acid groups previously formed on the CNT surface. In a typical procedure, 50 mg of O-CNTs were dispersed in 50 mL of toluene, using ultrasonication in a water bath for 60 min. Subsequently, 5 mL of a 10 wt% solution of ethylenediamine in toluene, 800 mg of dicyclohexylcarbodiimide, and 100 mg of dimethylaminopyridine were sequently added, and the mixture was stirred magnetically at 60℃ for 2 h. After the reaction, 50 mL of ethanol was added in order to dilute the unreacted ethylenediamine and the catalytic molecules. Amino-CNTs were obtained by filtration and washing with ethanol and water, three times each. The product was dried in a vacuum at 60℃ for 4 h [22].
Ma et al. [22] studied the amino-functionalization of UV/O3- treated CNTs. Surface functionalization was confirmed by FTIR and XPS. The results of static contact angle measurements indicated that the amino-CNTs contained both amine and amide groups on the surface, resulting in enhanced hydrophilicity, and therfore adhesivity, to the epoxy resin, as shown in Table 4.
Jin et al. [17] prepared dodecylamine-functionalized CNTs (amino-CNTs). As shown in Fig. 2, in the thermogrametric analysis (TGA) the temperature of 5% weight loss of P-CNTs was 735℃. The weight loss of the amino-CNT sample at this temperature was significantly higher, at 15.2%, which was mainly attributed to the decomposition of dodecyl groups. TEM images demonstrated that the outer shell of the amino-CNTs was covered
[Table 4.] Contact angles of various droplets on CNT substrate [22]
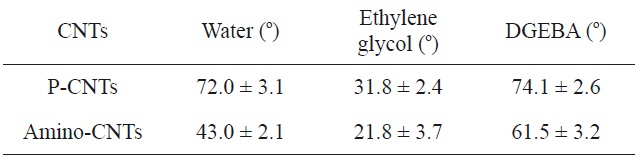
Contact angles of various droplets on CNT substrate [22]
with a thin layer of amorphous material, as shown in Fig. 3.
Yaping et al. [32] studied the effect of functionalization on epoxy/CNT nanocomposites. TEM results showed that amino- CNTs were efficiently dispersed in the nanometric-class microfibers in the epoxy matrix. The diameter of the amino-CNTs ranged from 20 to 30 nm, and the length reached several hundred microns. The amino-CNTs were evenly distributed in the epoxy resin, ensuring a greater interfacial strength, which is beneficial for improving the properties of the epoxy resin.
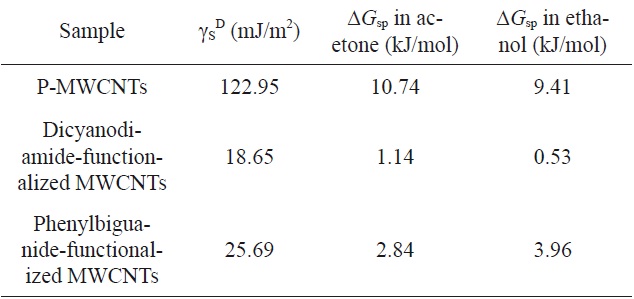
Dispersive surface energies (γS D) and specific free energies (ΔGsp) of different MWCNTs [29]
Luo et al. [29] investigated the effect of amino-functionalization on the interfacial adhesion of epoxy/CNT nanocomposites. As shown in Table 5, the grafting of amino-organics onto the surface of MWCNTs resulted in a reduction in dispersive surface energy,
Armstrong et al. [33] investigated improved performance of epoxy nanocomposites containing amine-functionalized CNTs. SEM images showed worm-like structures, and the nanotubes were thicker and appeared inflated. The images also showed a Y-junction where two CNTs were connected. The junctions, as well as the inflation of the amino-CNTs, would be expected to affect the properties of the final epoxy/CNT composites.
Yang and Gu [34] studied the effects of grafting triethylenetetramine to CNTs on the dispersion, the filler-matrix interfacial interactions, and the thermal properties of the epoxy nanocomposites. TEM images demonstrated the appearance of an extra phase on the MWCNT wall after chemical modification, indicating that the grafting reactions had successfully occurred. SEM images showed that the triethylenetetramine grafting could break up large agglomerations and produce individual MWCNTs.
2.2.2. Silane modification of CNTs
The silanization of CNTs is another method that has been used to enhance the interfacial adhesion between nanotubes and the matrix. The silane coupling agents most commonly used are organosilanes, which readily react with hydroxyl groups produced on the CNT surface using oxidation and/or reduction processes.
A general procedure for silane modification of CNTs is as follows. O-CNTs are dispersed in a silane solution via ultrasonication for 30 min, which is then added to a mixture of ethanol/ water (95:5 v/v). The reaction is conducted with stirring at 65℃ for 4 h. The resulting silanized CNTs are separated by filtration, washed with distilled water and acetone, and dried in a vacuum at 80℃ for 20 h [30].
Lee et al. [26] studied silane modification of O-CNTs using 3-aminopropyltriethoxysilane (APTES). The surface modification and subsequent interactions with epoxy resin were characterized by FT-IR spectroscopy and SEM, respectively. The SEM results showed that the dispersion and impregnation of silanized CNTs in the epoxy resin were improved compared to the O-CNTs.
[Table 6.] Element composition of MWCNTs by EDS analysis [30]
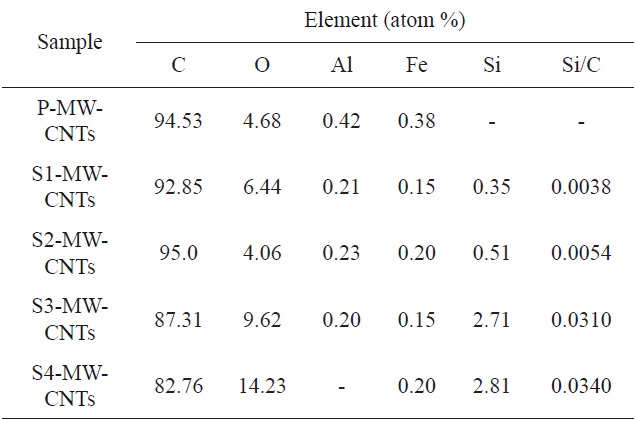
Element composition of MWCNTs by EDS analysis [30]
Kim et al. [30] showed the silanization of O-MWCNTs, again using APTES. The results of energy-dispersive X-ray spectroscopy (EDS) analysis confirmed the attachment of the silane molecules to the surface of the O-MWCNTs, as shown in Table 6.
Kuan et al. [35] used a free radical reaction to silane-functionalize CNTs. FT-IR was used to monitor the reaction between the coupling agent and the CNTs. Solid-state 29Si NMR spectroscopy revealed that the reactant components underwent a sol?gel reaction to form covalent bonds between the organic and inorganic phases.
2.2.3. Fluorination of CNTs
A general procedure for fluorinating CNTs is as follows. OMWCNTs (1 g), 15 mL of 2-methoxyethyl ether, and 3.1 mL of 4-fluoroaniline are added to a flame-dried three-necked roundbottomed flask fitted with a condenser. While maintaining a N2 atmosphere, 4 mL of amyl nitrate is added slowly. The mixture is stirred at room temperature for 1 h and then the temperature is raised to 70oC, followed by mixing for 3 h. The product is cooled, diluted with diethyl ether, filtered, and then washed with copious amounts of distilled water. The wet product is dried in a vacuum at 60℃ for 24 h [36].
Abdalla et al. [36] studied the link between the nature of the CNT surface modification and the quality of dispersion in an epoxy resin. Acid-treated and fluorinated MWCNTs were characterized by FT-IR and Raman spectroscopy, and SEM. The SEM images showed very good dispersion of the fluorinated CNTs in the epoxy matrix.
2.2.4. Epoxy functionalization of CNTs
A general procedure for epoxy functionalization of CNTs is as follows. O-CNTs (2.00 g) and 3.00 g of 4,4'-bis(2,3-epoxypropoxy) biphenyl (BP) epoxy resin are dispersed in 200 mL of tetrahydrofuran (THF) and then ultrasonicated in a 100 W bath sonicator at room temperature for 1 h. KOH (8.96 g) is added to the solution as a catalyst and the solution is refluxed at 70℃ for 6 h. The epoxy-functionalized CNTs are collected by filtration and then dried, resulting in a black powder [16].
Hsu et al. [16] functionalized CNTs using a liquid crystalline (LC) BP epoxy resin. The FT-IR spectra contained an epoxide ring peak, implying that not all of the rings opened and reacted during the functionalization process. TEM images of O-CNTs showed a rough and damaged surface, whereas those of epoxy functionalized CNTs showed a smoother surface.
2.2.5. Poly(glycidyl methacrylate) (PGMA)-grafted CNTs
A general procedure for the production of PGMA-grafted CNTs is as follows. CNTs, glycidyl methacrylate (GMA), and 2,2'-azobis-isobutyronitrile (AIBN) are mixed in a 6.2:1:1 molar ratio. The mixture is dispersed in N-methylpyrrolidone and sonicated at 65℃ in a N2 atmosphere for 2 h, and then stirred for 24 h. After the reaction, the PGMA-grafted CNT slurry is washed several times with acetone to remove all non-grafted GMA, filtered, and then dried in a vacuum for 24 h [37].
Teng et al. [37] studied the thermal conductivity of epoxy composites containing both functionalized CNTs and aluminum nitride. XPS and SEM were used to investigate P-MWCNTs and GMA-grafted MWCNTs. SEM images indicated that an interconnected macro-nano binary network structure was constructed between the aluminum nitride and MWCNT fillers.
2.2.6. Esterification of CNTs
A general procedure for the esterification of CNTs is as follows. O-CNTs (1 g), phenyl glycidyl ether (PGE) (molar ratio PGE/COOH = 3), and triphenyl phosphine (TPP) (molar ratio TPP/PGE = 0.2) are dispersed in 200 ml of THF. The esterification reaction is conducted with a refluxing solvent for 96 h. After centrifugation and extensive washing with THF to remove unreacted PGE and TPP, the esterified nanotubes are dried in a vacuum at 90℃ for 24 h [38].
Auad et al. [38] studied the esterification of both singlewalled CNTs (SWCNTs) and MWCNTs. TGA thermograms indicated that the organic mass attached to the carbon surface increased after the oxidation process because of the generation of carboxyl groups and increased further after esterification with PGE. Raman spectra demonstrated that the quality of the dispersion decreased in the following order: O-SWCNTs>P-SWCNTs> PGE-functionalized SWCNTs.
3. Preparation Methods for Epoxy/CNT Composites
The surface area of a CNT is several times greater than that of conventional fillers used in epoxy composites. This makes it difficult to homogenously disperse CNTs within the epoxy matrix and improve the interfacial bonding between the two components. Therefore, a key requirement for improved processing techniques for epoxy/CNT composites is the prevention of nanotube aggregation. Various investigations focusing on methods of mixing CNTs into the polymer matrix have been reported in the literature [39-48].
As previously mentioned, the principal methods for achieving dispersion of CNTs can be categorized as mechanical, physical, and chemical. Section 2 of this article dealt with chemical approaches to improving CNT dispersion. This section will focus on the mechanical methods currently under investigation, including ultrasonication and high-shear and high-impact mixing. It should be noted that mechanical methods do not permanently stabilize the dispersion [40].
Sonication is a commonly used technique for distributing CNTs in an epoxy matrix and involves the application of ultrasound energy to agitate particles in a solution. In the laboratory, it is usually achieved using an ultrasonic bath or an ultrasonic probe/horn. However, this technique is not easily scalable to industrial-level production, where other technologies, such as three-roll or ball mills, can assure a comparable high quality of mixing, together with the possibility of treating a large amount of material [39,41].
Recently, several methods have been developed for preparing epoxy/CNT composites with a high nanotube content, including the hot-press molding process, the mechanical densification method, and the layer-by-layer (LBL) method. In hot-press molding, a filtration system was employed to impregnate the epoxy resin into CNT buckypaper. In mechanical densification, vertically aligned CNT forests were densified followed by capillarity- induced wetting with epoxy resin. In the LBL method, the composites were formed on solid substrates by the sequential deposition of layers of oppositely charged polymers and CNTs [42].
The preparation methods for epoxy/CNT composites are discussed with respect to direct mixing processing and solution processing in the following sections.
A general procedure for the direct mixing process is as follows. CNTs are added to epoxy resin and dispersed at an elevated temperature by application of intense shear forces (such as sonication or magnetic stirring). A curing agent is added to the epoxy/CNT mixture and agitated using magnetic stirring. To remove entrapped air and voids, the mixture is degassed in a vacuum. Finally, the mixture is transferred to silicone rubber molds and cured using the usual temperature profile.
Rahatekar et al. [43] studied the dispersion of CNTs in bisphenol A epoxy resin using high-shear mixing at 200 rpm for 2 h. Samples were cured at 60℃ for 2 h and post-cured at 90℃ for 15 h. The optical microstructure showed a weak aggregation of CNTs as a result of unfavorable interactions with the epoxy matrix.
Gkikas et al. [40] used an ultrasonic mixer to disperse CNTs in epoxy resin. In order to avoid overheating and induction of defects on the CNT surfaces, the temperature of the mixture was kept low by submerging the container in an ice bath. Initial experiments were carried out for four different sonication time periods to thoroughly investigate the effect of the sonication conditions. The results showed that the duration and amplitude of the sonication process was of key importance for the dispersion of the CNTs in the epoxy resin.
Loos et al. [44] prepared suspensions of CNTs in epoxy resin with different amounts of block copolymers using a tip sonicator. SEM images showed that the dispersion of CNTs treated by block copolymers in the composites was enhanced.
Saw et al. [45] made transparent, electrically conductive, and flexible films by adding CNTs to the epoxy resin followed by sonication using a probe sonicator at room temperature. Optical micrographs showed that the short CNTs were well dispersed within the epoxy resin.
Gojny et al. [46] used a mini-calender and high-shear mixing
to enhance the dispersibility of CNTs in epoxy resin. TEM images suggested that the amino groups stabilized the CNT dispersion by forming stronger interactions with the epoxy matrix.
Schulz et al. [39] produced CNT suspensions using two common dispersion methods, sonication using a horn ultrasonicator and milling by means of a 3-roll mill. Sonication is often used to produce small batches, whereas milling is more favorable for achieving good dispersion for larger amounts of material. Light microscopy images showed that milling was able to produce a fine and uniform dispersion of CNTs, but short sonication times were not able to break up the initial nanotube agglomerates.
Jin et al. [17] prepared epoxy/amino-CNT composites using a sonicator. As shown in Fig. 4, SEM images demonstrated that the amino-CNTs were well dispersed in the epoxy matrix. TEM micrographs also showed that the functionalized CNTs were separated and dispersed uniformly in the epoxy matrix, as shown in Fig. 5.
A general procedure for solution processing of CNTs is as follows. CNTs are dispersed in a solvent using sonication and the resulting solution is added to epoxy resin and dispersed by the application of intense shear forces. The curing agent is then added to the epoxy/CNT mixture and magnetic stirring is used for further agitation. The resulting mixture is degassed at elevated temperature to eliminate the remaining solvent and trapped air. The composite resin is then molded in a steel mold and cured using the tracditional temperature profile.
Feng et al. [42] dispersed CNTs in
Prolongo et al. [47] reported dispersion of CNTs in chloroform using magnetic stirring at 45℃. Composites with different amino-CNTs contents were prepared with and without the pre-curing treatment. Field emission gun SEM (FEG-SEM) micrographs showed that the degree of dispersion achieved for the pre-cured samples was better than that for the non-treated samples.
Cividanes et al. [48] studied the dispersion of CNTs in acetone using an ultrasonicator bath and then mixing with epoxy resin. SEM micrographs showed that amine-functionalized CNTs had more disorganized microstructures, which led to greater dispersion of CNTs.
4. Mechanical and Electrical Properties
It is known that the mechanical performance of epoxy/CNT composites strongly depends on the uniformily of nanotube dispersion within the polymer matrix and the strong interfacial interactions between the two components [22,23,47]. It has been shown that functionalized CNTs are more readily dispersed in an epoxy matrix and have better interfacial interactions, which is beneficial for improving the properties of the composites [32].
Many researchers have reported that the formation of covalent bonding between functionalized CNTs and an epoxy matrix leads to more effective stress transfer and a denser crosslinked structure. This could limit the mobility of the matrix backbone and therefore improve the mechanical properties of epoxy composites [17].
Fracture toughness has been demonstrated to be improved by surface functionalization of CNTs, which was attributed to the improved interfacial bonding strength between the nanotubes and the epoxy matrix. This would lead to a suppression of debonding at the interface and crack propagation [49].
Many studies have shown that CNTs are able to elastically deform under relatively large stresses, both in tension and compression, leading to highly energy-absorbing processes. It has also been shown that the unique flexibility and geometric features of the CNTs contribute to continuous absorption of energy, resulting in increased elongation in the epoxy component [18,50].
Jin et al. [17] studied the mechanical interfacial properties of diglycidyl ether of bisphenol A (DGEBA) epoxy resin reinforced with amino-CNTs using critical stress intensity factor (
Farahani et al. [51] investigated the use of biotin-streptavidin interactions to reinforce epoxy nanocomposites containing functionalized CNTs. The improvement of the tensile modulus for epoxy/CNT composites with 1 wt% biofunctionalized CNT loading was 93% in a comparison to that of the neat epoxy resin, as shown in Fig. 7. The increased stiffness could be attributed
[Table 7.] Hardness of ef-CNT/epoxy/DDS composites [16]

Hardness of ef-CNT/epoxy/DDS composites [16]
to the proper dispersion, as well as beneficial orientation, of the nanotubes that may occur during the extrusion of the composite through the micronozzle.
Hsu et al. [16] assessed the mechanical properties of biphenyl liquid crystalline epoxy/CNTs composites. As shown in Table 7, the Vickers hardness of the neat epoxy resin was only 16.62 Hv, whereas that of the composite with 2.00 wt% epoxy-functionalized CNTs (ef-CNTs) was 63% higher, at 27.14 Hv. This was the result of homogeneous dispersion of the CNTs and improved rigidity and hardness via improved interfacial interaction with the epoxy matrix.
Luan et al. [19] studied the effect of pyrene-modified MWCNTs on the properties of epoxy composites. Fig. 8 shows the effect of MWCNT content on the impact toughness of the composites. Compared with the neat resin, the impact toughness values of P-CNT composite and poly(styrene-b-pyrene) (PS-b- PAH) modified CNT composite were improved by 33.09% and 127.94%, respectively, when the CNT content was increased to 0.6 wt%. SEM images of the epoxy/PS-b-PAH modified CNT composite indicated that the block copolymer modifier acted as a dispersant of the MWCNTs within the matrix.
Kwon et al. [52] studied the dispersion, hybrid interconnection, and heat dissipation properties of functionalized CNTs in epoxy composites. The mechanical characteristics of the composites were confirmed by measuring the mechanical strengths of completely interconnected quad flat packages (QFPs). Fig. 9 shows the pull strength data for the QFP solder joints for all epoxy/ CNT composites. In the case of DGEBA, the pull strengths of P-CNT, O-CNT, and amino-CNT composites were 6.6, 11.2, and 12.6 N, respectively. The pull strength of the DGEBA/amino- CNT composite was twice as high as that of the composite containing P-CNTs. This can be attributed to the strong covalent
bonds formed between the amino groups on the CNT surfaces and the epoxy matrix, which resulted in improved mechanical properties.
Xu et al. [53] studied the tensile strength of conducting epoxy/ polyaniline-grafted CNT (PANI-g-CNT) composites. As shown in Fig. 10, the tensile strength increased by 61% from 35.26 (neat epoxy) to 56.93 MPa, when the PANI-g-CNT content was raised from 0 to 1 wt%. This was attributed to the reaction of amino groups on the CNT surfaces with the epoxy matrix
during curing, providing interfacial adhesion for load transfer between the polymer and the nanotubes.
Xu et al. [23] reported the reinforcement of epoxy nanocomposites with poly-(2-hydroxyethyl methacrylate) (PHEMA)- grafted CNTs (PHEMA-g-CNTs). Fig. 11 shows the flexural moduli of the epoxy/PHEMA-g-CNT nanocomposites as a function of CNT content. The results revealed that the flexural modulus increased steadily with the amount of CNTs incorpareted, as a result of stronger interfacial interactions between the PHEMA-g-MWCNTs and the epoxy matrix, which enabled a more effective transfer of stress from the polymer to the CNTs.
Prolongo et al. [47] studied the flexural properties of epoxy/ amino-CNT composites prepared using a pre-curing treatment. As shown in Fig. 12, the epoxy resin reinforced with 0.40% CNTs exhibited an increase of 45% in flexural strength. However, at lower CNT contents, the composite with 0.25% CNTs, which was subjected to pre-curing thermal treatment, showed
improved mechanical properties, with a 58% increase in strength over the pristine epoxy resin. This indicated that the pre-curing treatment induced interfacial bonding, enabling effective stress transfer between the epoxy matrix and the amino-functionalized CNTs.
Guo et al. [54] investigated the effects of MWCNT addition and surface modification on the mechanical performance of epoxy/MWCNT composites. As shown in Fig. 13, the tensile strength of the composites improved with an increasing MWCNT addition. In addition, the fracture strain was also distinctly enhanced, implying that MWCNT loading not only elevatedle strength of the epoxy matrix but also increased the fracture toughness.
Kim and Park [55] studied the effect of amino-MWCNTs on the mechanical interfacial properties of epoxy nanocomposites. The impact strengths of epoxy/amino-MWCNT composites with different nanotube content are shown in Fig. 14. The impact strengths of the nanocomposites were remarkably improved with increasing the amino-MWCNT content up to 0.6 wt%.
[Table 8.] Electrical conductivity of various PAni-CNTs/EP composites [20]
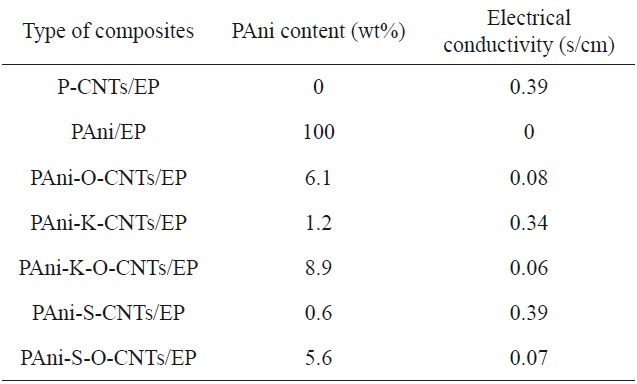
Electrical conductivity of various PAni-CNTs/EP composites [20]
CNTs exhibit excellent electrical properties (of the order of 103-104 S/cm). Their high conductivity makes them excellent candidates for producing conductive polymer composites. The formation of an electrically conductive nanotube pathway in a polymer composite is characterized by the percolation threshold, which is the minimum concentration of conductive filler required to form a three-dimensional network [52,56]. It is well known that the percolation thresholds of polymer/CNT composites in general depend on the aspect ratio of the conducting fillers and degree of dispersion of CNTs. The higher the aspect ratio is, the lower the percolation threshold. Experimental results have shown that homogenous dispersion and alignment of CNTs in the matrix increases the electrical conductivity of CNT-filled composites. Moreover, the conductivity continues to increase with an increasing CNT content, even after the percolation threshold has been reached [42,45].
Park et al. [20] studied the effects on the electrical properties of epoxy/CNT composites of coating the CNTs with polyaniline (PAni-CNTs). As shown in Table 8, the epoxy/P-CNT composite exhibited the highest conductivity among those tested, with the conductivity decreasing with an increasing PAni coating thickness. The epoxy/PAni-coated, potassium persulfate treated O-CNTs (PAni-K-O-CNTs) composite showed the lowest electrical conductivity among the composites as a result of the presence of a thicker electrically insulating layer.
Liu et al. [57] reported MWCNT-reinforced epoxy composites with polyethylenimine as a dispersant. Fig. 15 shows the electrical conductivities of the epoxy/MWCNT-polyethylenimine composites. The covalently modified composites were 10000 times more resistive than their non-covalent counterparts. The storage moduli of the composites containing covalently functionalized nanotubes increased relative to the non-covalent composites, as a result of the stronger polymer-nanotube interactions.
Bai and Allaoui [58] studied the effect of CNT length and aggregate size on the electrical properties of epoxy/CNT composites.
The insulator-to-conductor transition occurred at 0.5 wt% P-MWCNTs, as shown in Fig. 16.
Lee et al. [26] studied the effects of silane modification of CNTs on the electrical properties of epoxy/carbon/CNT threephase composites. Fig. 17 shows the volume resistivities of the epoxy/carbon/unmodified, oxidized, and silanized CNT composites, which were 2.8, 1.5, and 0.6 Ω·cm, respectively. The lower volume resistivity of the silanized composites may be a result of the formation of a continuous network structure caused by homogeneous dispersibility of CNTs between the carbon fibers.
Xu et al. [53] studied the electrical properties of conducting epoxy/PANI-g-MWCNT composites. The electrical conductivity for neat epoxy is 2.848×10-13 S/cm. Incorporation of 1.0 wt% PANI-g-MWCNTs increased this by seven orders of magnitude to 1.975×10-6 S/cm. This increase in electrical conductivity implies that the percolation threshold for the composites was between 0.25 and 1.0 wt% of PANI-g-MWCNT content. Because of the encapsulation by the swelling PANI coatings,
PANI-g-MWCNTs could not aggregate and were homogenously dispersed in the epoxy resin, resulting in formation of electrical networks.
Martone et al. [41] studied the electrical conductivity of epoxy/MWCNT nanocomposites. Fig. 18 shows the electrical conductivities of the nanocomposites as a function of MWCNT content. A percolative transition between the insulating and conducting behaviour occurred above 0.1 wt% MWCNTs.
Saw et al. [45] prepared transparent, electrically conductive, and flexible films from epoxy/MWCNT composites. Fig. 19 shows the electrical conductivities of the composite films plotted as a function of CNT content for two different types of CNT. The conductivity increased with increasing CNT content and there was a jump in conductivity by almost four orders of magnitude when the CNT content reached 1 wt%. After the percolation threshold was reached, the electrical conductivity showed a further gradual increase.
In this paper, we have reviewed the surface modification of CNTs, processing methods, and mechanical and electrical properties of epoxy/CNT composites. The surfaces of CNTs were treated using oxidation and chemical treatment methods to improve the dispersion stability of CNTs and interactions between them and epoxy resins. The electrical and mechanical properties of the composites were significantly improved by the addition of CNTs. CNT-based epoxy composites are of great interest as multifunctional high-performance materials for use in aircraft and electronic products.
![Elemental composition of CNTs before and after acid treatment [17]](http://oak.go.kr/repository/journal/11894/HGTSB6_2013_v14n1_1_t001.jpg)
![Raman spectra of CNTs before and after acid oxidation [17]. PCNTs: pristine carbon nanotubes, O-CNTs: oxidized CNTs.](http://oak.go.kr/repository/journal/11894/HGTSB6_2013_v14n1_1_f001.jpg)
![Raman spectroscopy of untreated and treated MWCNTs [30]](http://oak.go.kr/repository/journal/11894/HGTSB6_2013_v14n1_1_t002.jpg)
![Chemical composition of the functional groups on surface of P-MWCNTs, O-MWCNTs and H2O-A-MWCNTs [31]](http://oak.go.kr/repository/journal/11894/HGTSB6_2013_v14n1_1_t003.jpg)
![Contact angles of various droplets on CNT substrate [22]](http://oak.go.kr/repository/journal/11894/HGTSB6_2013_v14n1_1_t004.jpg)
![Thermogrametric analysis thermograms of CNT samples [17]. P-CNTs: pristine carbon nanotube, O-CNTs: oxidized CNTs, amino-CNTs: dodecylamine-functionalized CNTs.](http://oak.go.kr/repository/journal/11894/HGTSB6_2013_v14n1_1_f002.jpg)
![Transmission electron microscopy image of amino-CNTs [17]. amino-CNTs: dodecylamine functionalized carbon nanotubes.](http://oak.go.kr/repository/journal/11894/HGTSB6_2013_v14n1_1_f003.jpg)
![Dispersive surface energies (γS D) and specific free energies (ΔGsp) of different MWCNTs [29]](http://oak.go.kr/repository/journal/11894/HGTSB6_2013_v14n1_1_t005.jpg)
![Element composition of MWCNTs by EDS analysis [30]](http://oak.go.kr/repository/journal/11894/HGTSB6_2013_v14n1_1_t006.jpg)
![Scanning electron microscope spectroscopy images of the fracture surface in DGEBA/amino-CNT/DDM composites [17]. amino-CNTs: dodecyl amine functionalized carbon nanotubes.](http://oak.go.kr/repository/journal/11894/HGTSB6_2013_v14n1_1_f004.jpg)
![Transmission electron microscopy photographs of DGEBA/amino- CNT/DDM composites [17]. amino-CNTs: dodecylamine functionalized carbon nanotubes.](http://oak.go.kr/repository/journal/11894/HGTSB6_2013_v14n1_1_f005.jpg)
![KIC values of DGEBA/CNT composites; (a) DGEBA/DDM, (b) DGEBA/P-CNT/DDM, (c) DGEBA/O-CNT/DDM, (d) DGEBA/amino-CNT/ DDM [17]. P-CNTs: pristine carbon nanotubes, O-CNTs: acid treated CNTs, amino-CNT: dodecylamine functionalized CNTs.](http://oak.go.kr/repository/journal/11894/HGTSB6_2013_v14n1_1_f006.jpg)
![Modulus of epoxy/CNT nanocomposites [51]. (a) Neat epoxy, (b) 0.5 wt%-purified SWCNTs/epoxy, (c) 0.5 wt%-biofunctionalized SWCNTs/ epoxy, (d) 1 wt%-purified SWCNTs/epoxy, (e) 1 wt%-biofunctionalized SWCNTs/epoxy. SWCNT: single-walled carbon nanotube.](http://oak.go.kr/repository/journal/11894/HGTSB6_2013_v14n1_1_f007.jpg)
![Hardness of ef-CNT/epoxy/DDS composites [16]](http://oak.go.kr/repository/journal/11894/HGTSB6_2013_v14n1_1_t007.jpg)
![Effect of CNT content on impact toughness of epoxy/CNT composites [19]. PS-b-PAH-CNTs: poly(styrene-b-pyrene) (PS-b-PAH) modified carbon nanotubes.](http://oak.go.kr/repository/journal/11894/HGTSB6_2013_v14n1_1_f008.jpg)
![Pull strengths of the quad flat package leads [52]. (a) Epoxy/PCNTs, (b) epoxy/A-CNTs, (c) epoxy/Amine-CNTs. P-CNTs: pristine carbon nanotubes, A-CNTs: acid treated CNTs, D-CNTs: amine functionalized CNTs.](http://oak.go.kr/repository/journal/11894/HGTSB6_2013_v14n1_1_f009.jpg)
![Tensile strength of epoxy composites as a function of PANI-g- MWCNT content [53]. PANI-g-MWCNTs: polyaniline-grafted multiwalled carbon nanotubes.](http://oak.go.kr/repository/journal/11894/HGTSB6_2013_v14n1_1_f010.jpg)
![Flexural modulus of epoxy/CNT nanocomposites [23]. PHEMAg- CNTs: poly(2-hydroxyethyl methacrylate) grafted multi-walled carbon nanotubes.](http://oak.go.kr/repository/journal/11894/HGTSB6_2013_v14n1_1_f011.jpg)
![Ultimate flexural strength neat epoxy resin, non-treated and thermal pre-cured carbon nanotube (CNT)/epoxy composites [47]. (a) DGEBA/DDS, (b) DGEBA/CNT/DDS (0.25 wt% CNTs), (c) DGEBA/CNT/ DDS (0.25 wt% CNTs, pre-cured), (d) DGEBA/CNT/DDS (0.4 wt% CNTs), (e) DGEBA/CNT/DDS (0.4 wt% CNTs, pre-cured).](http://oak.go.kr/repository/journal/11894/HGTSB6_2013_v14n1_1_f012.jpg)
![Effects of MWCNT content on tensile strength of epoxy/MWCNT composites [54]. MWCNTs: multi-walled carbon nanotubes.](http://oak.go.kr/repository/journal/11894/HGTSB6_2013_v14n1_1_f013.jpg)
![Impact strength of epoxy/MWCNT nanocomposites with different amino-MWCNT content [55]. amino-MWCNTs: amine functionalized multi-walled carbon nanotubes.](http://oak.go.kr/repository/journal/11894/HGTSB6_2013_v14n1_1_f014.jpg)
![Electrical conductivity of various PAni-CNTs/EP composites [20]](http://oak.go.kr/repository/journal/11894/HGTSB6_2013_v14n1_1_t008.jpg)
![Electrical conductivity and modulus of epoxy/CNT-PEI composites [57]. CNT-PEIs: polyethylenimine grafted multi-walled carbon nanotubes.](http://oak.go.kr/repository/journal/11894/HGTSB6_2013_v14n1_1_f015.jpg)
![Evolution of conductivity of epoxy/carbon nanotube (CNT) composites as a function of CNT content for three treatments [58].](http://oak.go.kr/repository/journal/11894/HGTSB6_2013_v14n1_1_f016.jpg)
![Volume resistivities of epoxy/carbon/unmodified, oxidized, and silanized carbon nanotube (CNT) composites [26].](http://oak.go.kr/repository/journal/11894/HGTSB6_2013_v14n1_1_f017.jpg)
![Conductivity of carbon nanotube (CNT)/epoxy composites prepared by sonication aided dispersion at 120℃ vs. CNT content [41].](http://oak.go.kr/repository/journal/11894/HGTSB6_2013_v14n1_1_f018.jpg)
![Electrical conductivity of epoxy/multi-walled carbon nanotubes (MWCNT) composites as a function of MWCNT content [45].](http://oak.go.kr/repository/journal/11894/HGTSB6_2013_v14n1_1_f019.jpg)