The root bark of Ulmus davidiana var. Japonica (Ulmi Radicis cortex, URC) is a medicinal herb used for promoting diuresis and treating dampness. In Korea, URC has long been used as an efficacious therapy for inflammation, burns, frostbite and skin diseases such as eczema and psoriasis.
In the present study, we used 1-fluoro-2,4- dinitrofluorobenzene (DNFB)-induced contact dermatitis (CD) mouse model to investigate the antiallergic and the anti-inflammatory effects of URC on skin lesion, histopathological changes and specific antibody production.
URC treatment, 10 mg/mL, effectively inhibited skin lesions induced by repeated paintings with DNFB. In the histopathological observation, topical application of URC inhibited spongiosis. In addition, URC lowered the production levels of total immunoglobulin and IgG2a in serum.
These data indicate that URC has an anti-inflammatory effect that produces an improvement of skin lesions in CD mice.
In Korea, the root bark of
Contact dermatitis (CD) has an important economic and occupational health impact on society. CD presents as an inflammatory response to specific agents such as nickel and involves both skin resident cells and activated immune cells [6]. A widely used animal model of human CD is the delayed type hypersensitivity response to haptens such as dinitrofluorobenzene (DNFB) and dinitrochlorobenzene (DNCB) in mice [7]. Repeated applications of DNFB or DNCB are well known to induce typical features of CD, such as swelling, erythema and scale in the inflamed area [8].
Based on this background, we investigated the effects of URC by using a mouse model of CD. In the present study, we investigated the effects of URC on skin lesions, histopathological changes of tissues, and levels of antibodies in serum.
(DNFB) 1-fluoro-2,4-dinitrofluorobenzene, olive oil, and goat anti-mouse polyvalent antibody were purchased from Sigma-Aldrich (St. Louis, MO, USA). Goat anti-mouse polyvalent antibody, goat anti-mouse IgG1 antibody and goat anti-mouse IgG2a antibody were purchased from Southern Biotech (Birmingham, AL, USA).
Fifty g of URC were extracted using 99.9% methanol for 24 h. The extract was filtered and evaporated under reduced pressure by using a vacuum evaporator (Eyela, Japan). The condensed extract was then lyophilized. Finally, 3.57 g of lyophilized powder was obtained (yield; 7.1%).
Male balb/c mice (6 week old) were purchased from Samtaco (Incheon, Korea). The mice were housed under specific pathogen-free conditions with a 12 h light/dark cycle and free access to standard rodent food and water. All animal experiments were approved by our Animal Care and Use Committee and were performed according to institutional guidelines (PNU-2011-000406).
2.4. Induction of CD and experimental design
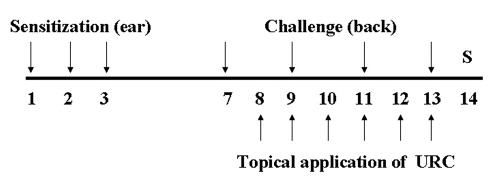
Mice were sensitized by painting with 30 ㎕of DNFB (0.1%, v/v) in acetone:olive oil (AOO, 4:1) on the dorsum of each ear for three consecutive days. Three days after sensitization, the dorsa of the mice were shaved. Four days after sensitization, the mice were challenged by painting the shaved dorsa with 50 ㎕ of DNFB (0.2%, v/v) in AOO every two days. URC was dissolved in ethanol, was then filtered using a syringe filter (0.45 ㎛), and was finally diluted in AOO (ethanol:AOO, 4:1). Ten mg/mL of URC solution was painted on the shaved dorsa for 6 days. Naive animals (Naive) were treated with the vehicle and was painted with the vehicle (n = 6). Control animals (CTL) were sensitized and challenged with DNFB and then painted with the vehicle (n = 8). URC-treated animals were sensitized and challenged with DNFB and then painted with 10 mg/mL of URC solution (n = 8). The experimental design is shown in (Fig 1)
2.5. Measurement the degree of skin lesion
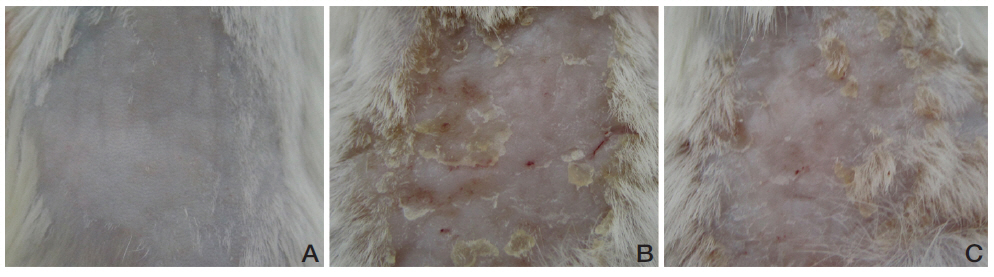
At the end of experiment, in order to observe the overall degree of CD, we sacrificed the mice and observed the skins of the dorsa by using a digital camera (Olympus, Japan).
2.6. Histopathological examination
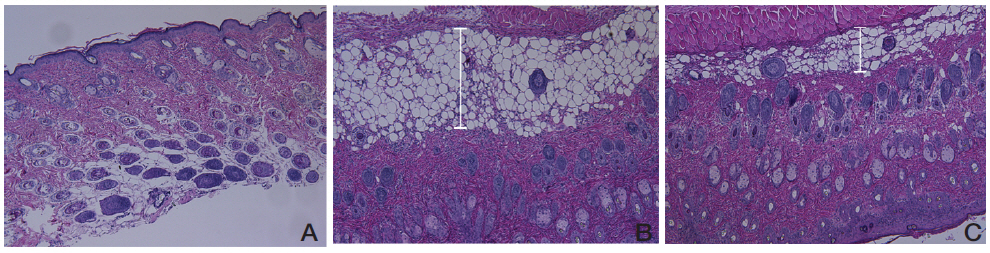
After measurements of the thicknesses and the weights of the ears, ear tissues were resected and embedded in paraffin. Sections were stained with hematoxylin and eosin (H&E) for histopathological observations such as immune cell infiltration and spongiosis. Stained tissues were observed using a light microscope (x100).
2.7. Measurement of immunoglobulin production
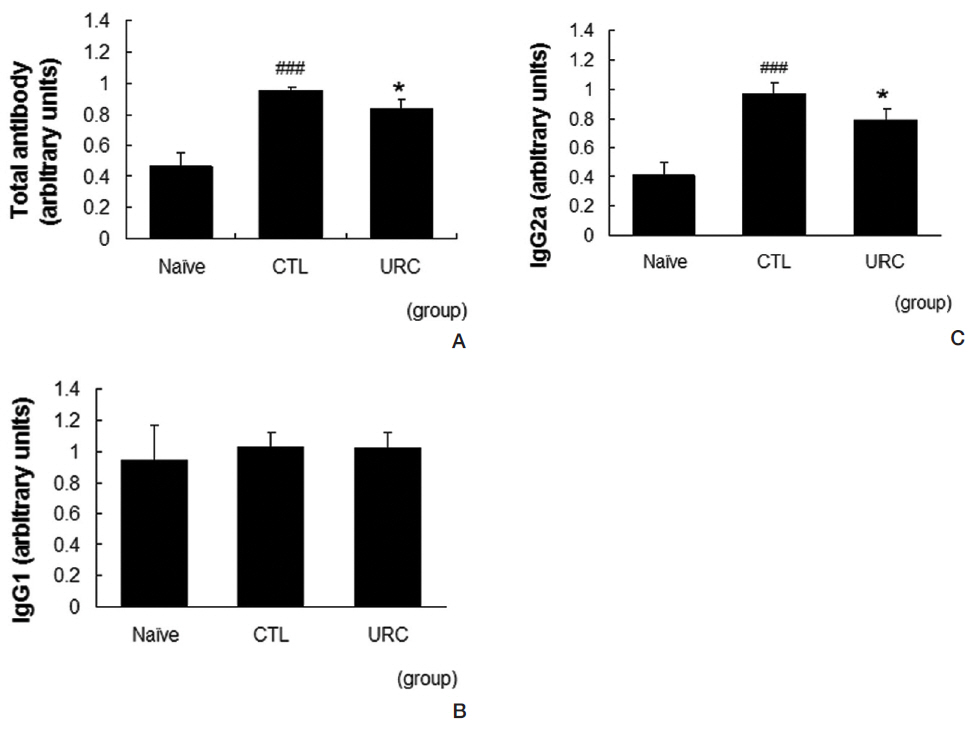
The levels of total antibody, IgG1 and IgG2a in serum were measured by using an enzyme-linked immunosorbent assay (ELISA). Plates with 96 well (Nunc, USA) were coated with goat anti-mouse polyvalent antibody (Sigma-Aldrich, 1:1000) at RT for 3 h and incubated at 4℃ overnight. After the plate had been washed, 100 ㎕ of diluted serum (1:20 in phosphate-buffered saline, PBS) was added to each well, and the plates were incubated at RT for 2 h. After the plate had been washed again, alkaline phosphatase-conjugated antimouse immunoglobulin was added, and the plates were incubated at RT for 1 h. After incubation, para-nitrophenyl phosphate (p-NPP) was added. The optical density (OD) was measured at 405 nm by using a microplate spectrophotometer (BioTek, VT, USA). Secondary antibodies used were goat anti-mouse polyvalent antibody (1:1000), goat anti-mouse IgG1 antibody (1:1000) and goat anti-mouse IgG2a antibody (1:1000). The serum obtained from another experiment was used as a standard serum and all the titers were calculated as values relative to that serum.
All statistical comparisons were made with the Student's
3.1. Effects of URC on the degree of skin lesions in CD
mice
URC improved skin lesions in CD mice. Repeated painting with DNFB resulted in skin lesions of CD such as erythema, scaling and crust (Fig 2B). The group treated with 10 mg/mL of URC showed diminished skin lesions compared to the vehicle-treated control group (Fig 2C).
3.2. Effects of URC on histopathological changes in CD mice
No abnormal changes in skin tissues were observed in the naive group (Fig 3A). The epidermis in the control group showed hyperplasia, and significant edema and spongiosis. In addition, marked infiltrations of mononuclear cells were observed in the dermis (Fig 3B). The URC-treated group showed diminished hyperplasia, edema and spongiosis compared to the non treated control group (Fig 3C).
3.3. Effects of URC on production levels of antibodies in CD
mice
URC lowered the production levels of total antibodies and IgG2a in serum. Repeated applications of DNFB resulted in increased levels of total antibodies and IgG2a in serum. In our model, the level of IgG1 in the control group was almost the same as that in the naive group. Treatment with 10 mg/mL of URC significantly suppressed the increases in the total antibody and the IgG2a levels (Fig 4A, C). The level of IgG2a was not affected by topical application of URC (Fig 4B).
Occupational skin diseases are the most common workrelated disorders worldwide. The most common cause of occupational skin diseases is CD [9]. The main etiologic treatment of CD is avoidance of the contact allergen, but in some cases, especially in occupational diseases, elimination of the offending agent is impossible. For this reason, therapy such as the use of corticosteroids, is rendered to assuage the inflammatory component [10, 11]. Because corticosteroids have some adverse effects, their usages have been restricted, so many of scientists are investigating complementary medicine and alternatives to corticosteroids. Recently, some research has focused on the use of medicinal plants as complementary medicine and as alternatives to corticosteroids, possibly because their low cost and safety [12]. In Korea, many medicinal herbs such as
In the present study, we showed the anti-allergic action of URC by using a mouse model of CD. Topical application of URC suppressed allergic reactions in the DNFB-induced CD model for mice. Skin lesions, such as erythema, crust and scaling, were diminished by topical application of URC (Fig. 2). Epidermal hyperplasia, which was related to inflammatory reactions with resident cells such as fibroblasts and keratinocytes, was slightly decreased in the histopathological observations (Fig 3) In addition, epidermal edema and spongiosis were diminished by topical application of URC (Fig 3) Intercellular epidermal edema is well known to be able to induce spongiosis, and is recognized as the microscopic hallmark of an inflammatory skin disease such as CD [16]. These data imply that epidermal spongiosis, edema and inflammatory cell infiltration result in skin lesions such as erythema, scaling and crust, and that the use of URC effectively prevented these inflammatory reactions in DNFBinduced CD.
Specific antibodies are well known to be involved in the pathophysiology of CD. In our results, repeated paintings with DNFB elevated the serum levels of total immunoglobulin and IgG2a. Topical application of URC effectively prevented elevations of total immunoglobulin and IgG2a in serum (Fig 4) These results imply that URC acts as an anti-inflammatory agent, and results in an improvement of skin lesions in CD mice. Considering that IgG2a is involved in the Th1 skewing reaction, these data could be a clue that URC can regulate the Th1/Th2 imbalance.
Taking these data together, we believe that URC can be used to treat patients with CD and may be useful as a complementary medicine or as an alternative to corticosteroids.
This study was designed to investigate the anti-allergic effects of URC by using a DNFB induced CD mouse model. We showed that URC could reduce skin lesions in inflamed tissues, epidermal spongiosis, and edema in histopathological observations. We also showed that URC could lower the production levels total antibodies and IgG2a in serum. These data suggest that URC can be used to treat patients with allergic skin diseases and that related mechanisms are involved in the anti-inflammatory action on the Th 1 skewing reaction.