This study was performed to analyze the single-dose toxicity of D-amino acid oxidase (DAAO) extracts.
All experiments were conducted at the Korea Testing & Research Institute (KTR), an institution authorized to perform non-clinical studies, under the regulations of Good Laboratory Practice (GLP). Sprague-Dawley rats were chosen for the pilot study. Doses of DAAO extracts, 0.1 to 0.3 cc, were administered to the experimental group, and the same doses of normal saline solution were administered to the control group. This study was conducted under the approval of the Institutional Animal Ethics Committee.
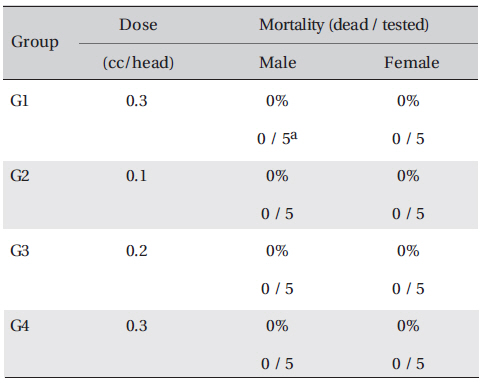
In all 4 groups, no deaths occurred, and the LD50 of DAAO extracts administered by IV was over 0.3 ml/kg.
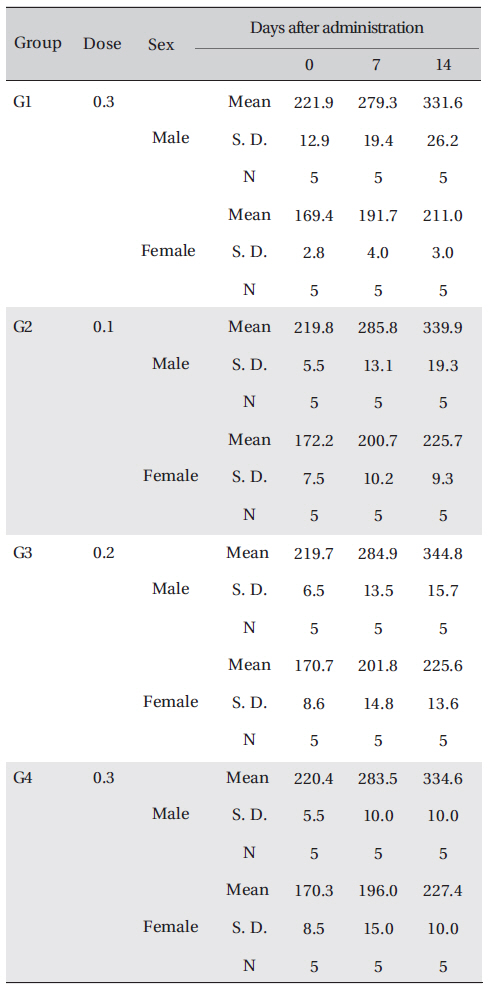
No significant changes in the weight between the control group and the experimental group were observed.
To check for abnormalities in organs and tissues, we used microscopy to examine representative histological sections of each specified organ, the results showed no significant differences in any organs or tissues.
The above findings suggest that treatment with D-amino acid oxidase extracts is relatively safe. Further studies on this subject should be conducted to yield more concrete evidence.
In mammals, DAAO is found at the highest concentrations in the kidneys, liver, and brain. In addition, DAAO catalyzes the oxidative deamination of a wide range of
DAAO was first described by Krebs in 1935 [3] and has been found to be one of the most important enzymes for the maintenance of proper levels of
The main role of DAAO in mammalian kidneys and liver cells is the detoxification of endogenous
In recent years, the important role of DAAO in maintaining the necessary levels of
DAAO plays an important role in regulating the levels of
Taken together, DAAO has potential as a novel therapeutic to treat various neural and psychiatric disorders. However, before clinical experiments can be performed, toxicity tests need to be conducted. Thus, this experiment was conducted to verify the toxicity of DAAO.
The current research trend for single-dose toxicity testing of extracts is to study acute and subacute toxicity through Good Laboratory Practice (GLP). All the experiments for this research were conducted under the GLP at the Korea Testing & Research Institute (KTR), an institution authorized to perform non-clinical studies.
The DAAO (0.1-0.3 cc, Sigma-Aldrich, St. Louis, MS, USA) extract was prepared in a clean room adhering to Korea- Good Manufacturing Practice(K-GMP) in a lab at the Korean Pharmacopuncture Institute. After the mixing process with pure water, the pH was controlled to between 7.25- and 7.35. NaCl was added to make a 0.9% isotonic solution. The completed extract was stored in a refrigerator.
The animals used in this study were 6-week-old Sprague- Dawley rats. The mean weights of the rats were 200.8-233.9 g, and 156.7-183.4 g for the male and female rats, respectively. For all animals, a visual inspection was done and all animals were weighed using a CP3202S system (Sartorius, Germany). After 7 days of acclimatization, the rats' general symptoms and changes in weight were recorded. No abnormalities were found.
The temperature of the lab was 22 ± 3°C and the humidity was 50 ± 20%. Enough food (Cargill Agri Purina) and UV- filtered water were provided.
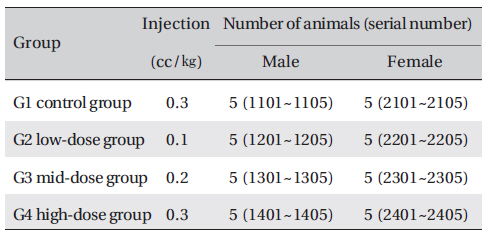
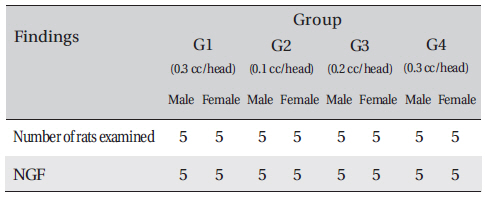
Groupings were done after 7 days of acclimatization. Animals were selected if their weights were close to the mean weight. In total, 20 male rats and 20 female rats were selected. The animals were distributed into 4 groups (5 mice per group) as follows (Table 1)
The expected dose for
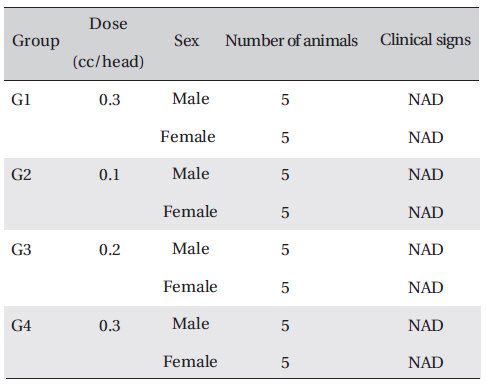
On the day of dosing (day 0), the general symptoms (types of toxic symptoms, revealing time, recovering time-,etc.) and the mortality were examined 30 min, and 1, 2, 3, and 4 h after the injection. From the 1st day to 14th day of treatment, the general symptoms were examined once a day.
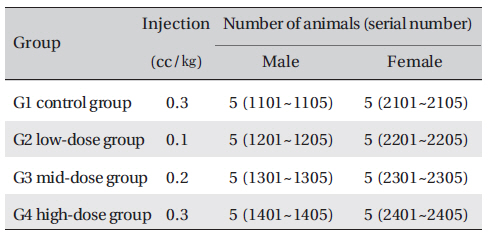
Number of animals
The weights were measured immediately before treatment, and at 7 and 14 days after treatment.
After the termination of observation, all surviving animal organs and tissues were visually inspected and examined by microscopy.
The weight results from the experiment were analyzed by using SPSS (version 10.0). Levene's test was conducted to evaluate the homogeneity of the variance and the significance. The One-way ANOVA test was conducted when a homogeneity of the variance was recognized, and the Scheffe's test was conducted post-hoc.
In this study, no deaths or abnormalities occurred in any of the groups, and the LD50 of the DAAO extracts administered via IV was over 0.3 ml/kg (Table 2), (Table 3) In addition, no changes in weight were observed in any of the groups (Table 4) Finally, no meaningful changes in necropsy were noted, and histopathological examination of all of Group 1 (0.3 cc/head) found no significant changes related to injections in the brain, lungs, liver, kidneys and spinal cord (Table 5)
Paul et al. did a study on the role of
Smith et al. did a study on the therapeutic potential of DAAO inhibitors. DAAO is a flavoenzyme that degrades
Zhao et al. did a study on the potential role of DAAO in neuropathic pain in a rat model of tight L5/L6 spinal nerve ligation and showed that spinal DAAO contributed significantly to the development of central sensitizationmediated pain, suggesting that DAAO may be an important molecular target for the treatment of chronic pain of neuropathic origin [11]. Verrall et al. did a study on the neurobiology of DAAO, it's involvement in schizophrenia, and the therapeutic value of DAAO inhibition. That study characterized DAAO as an enzyme that degraded the NMDA-R coagonist
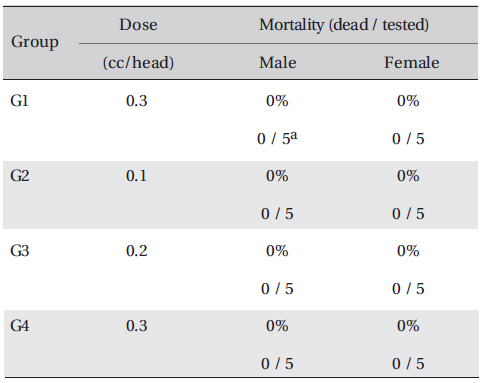
Mortality
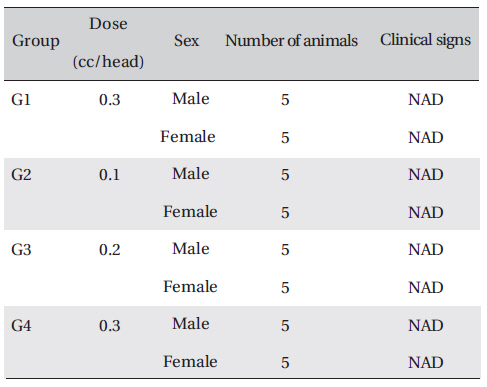
Clinical signs
[Table. 4] Body weights in grams
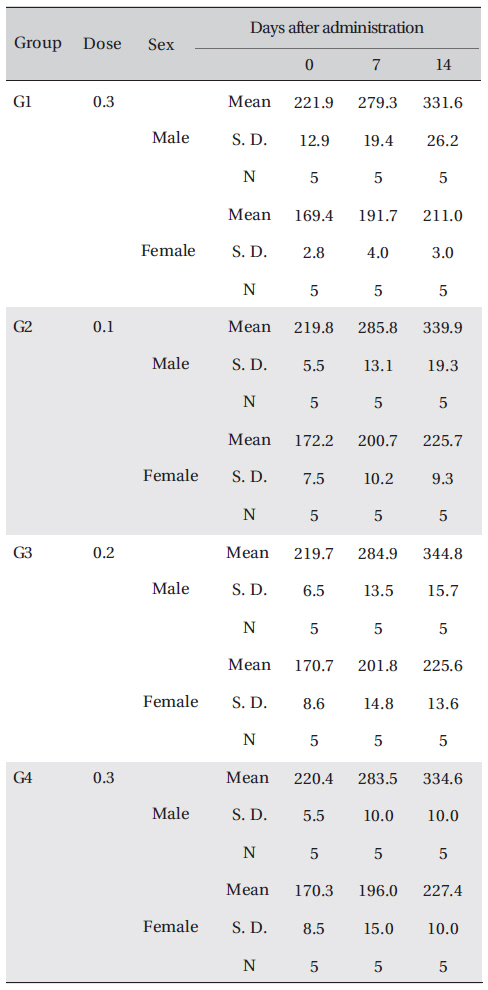
Body weights in grams
Necropsy findings
To assess the toxicity of DAAO, we need to study its acute and chronic harmful effects and its relations with the capacity-reaction more, and animal testing is the most fundamental and basic way to perform safety assessments [13]. The Korea Food & Drug Administration has testing protocol guidelines for the study of toxicity [14], and all experiments should be conducted following Good Laboratory Practice (GLP) regulations.
In this study, the LD50
The objective of this study was to analyze the single-dose toxicity of DAAO extracts. All experiments were conducted under the regulations of Good Laboratory Practice (GLP) at the Korea Testing & Research Institute (KTR), an institution authorized to perform non-clinical studies.
The results showed that administration of 0.3-ml/kg DAAO extracts did not cause any changes in weight and did not result in any mortalities which indicates that DAAO administration can be used as a safe treatment.