Evaluations of the in-vitro anti-bacterial activities of aqueous extracts of Acacia catechu (L.F.)Willd, Castanea sativa, Ephedra sinica stapf and Shilajita mumiyo against gram-positive bacteria (Staphylococcus aureus, Streptococcus pneumonia) and gram-negative bacteria (Escherichia coli, klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa) are reasonable since these ethnomedicinal plants have been used in Persian folk medicine for treating skin diseases, venereal diseases, respiratory problems and nervous disorders for ages.
The well diffusion method (KB testing) with a concentration of 250 μg/disc was used for evaluating the minimal inhibitory concentrations (MIC). Maximum synergistic effects of different combinations of components were also observed.
A particular combination of Acacia catechu
(L.F.) Willd, Castanea sativa, Ephedra sinica stapf and
shilajita mumiyo extracts possesses an outstanding
anti-bacterial activity. It's inhibiting effect on
microorganisms is significant when compared to the
control group (P < 0.05). Staphylococcus aureus was the
most sensitive microorganism. The highest antibacterial
activity against gram-positive bacteria
(Staphylococcus aureus, Streptococcus pneumonia) or
gram-negative bacteria (Escherichia coli, Klebsiella
pneumonia, Proteus mirabilis and Pseudomonas
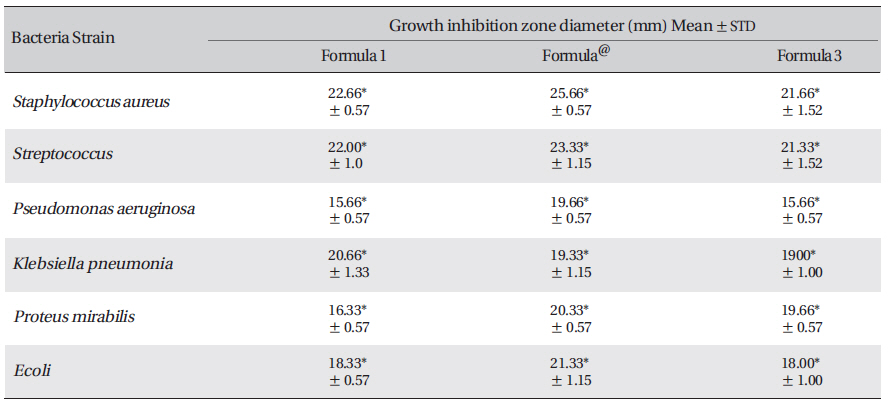
aeruginosa) was exerted by formula number 2 (Table
The results reveal the presence of antibacterial activities of Acacia catechu, Castanea sativa husk, Ephedra sp. and Mumiyo against gram-positive and gram-negative bacteria. Synergistic effects in a combined formula, especially in formula number 2 (ASLANⓡ) can lead to potential sources of new antiseptic agents for treatment of acute or chronic skin ulcers. These results considering the significant antibacterial effect of the present formulation, support ethno-pharmacological uses against diarrheal and venereal diseases and demonstrate use of these plants to treat infectious diseases.
The use of plants for healing purposes predates human history, and even animals have enjoyed such benefits. Self-medication through ingestion or topical application of non-nutritional substances such as bark or soil occurs in a variety of animal species like bears, cattle, birds and primates. Studies of African apes, in particular, have provided evidence of situation-specific ingestion of plants, such as infrequent intake of plant species which are not a regular part of the diet for assumed medicinal purposes, for example, a parasitic infection [1, 2].
Although new synthetic drugs are important, still more than 25% of drugs in developed countries are derived (directly or indirectly) from plants [3]. However, as sources of drugs, plants have not been utilized properly [4]. In rural areas, there are additional cultural factors that encourage the use of botanicals, such as the environment and culture, "man earth relationship." People believe that where an area gives rise to a particular disease, it will also support plants that can be used to cure it [5]. In Iran has a very distinguished past in traditional medicine. A significant ancient heritage is the experience of people with using plant medicines for health improvement [6]. In the recent past, the use of herbal medicine through the world has exceeded two to three times the use of conventional drugs (WHO) [7].
Plants synthesize numerous compounds called primary metabolites that are essential to their existence. These include proteins, lipids and carbohydrates that are used for subsistence and reproduction, not only by the plant itself but also by the animals that eat them. In addition, plants synthesize an extraordinary range of other compounds called secondary metabolites whose functions are from being unanimously accepted. Many secondary metabolites are "antibiotics" as they protect plants against fungi, bacteria, animals and even other plants. Plants contain substances that can be harmful to some animals or microorganisms because they fight disease and protect against herbivores. [8, 9].
A single herb may have several pharmacological actions, but only at sufficient concentrations. Synergy among components of different medicinal plants will enhance anti-bacterial activities and can be an effective strategy for fighting drug-resistant microorganisms [10]. These plants were used as folk medicine for the treatments of various ailments in Persian tribes. Ethnobotanical data (local name, mode of preparation, medicinal uses) were collected through interviews and discussions with tribal practitioners in their local language. This study was undertaken to evaluate the anti-bacterial activities of herbal medicines used in Persian folk medicine, to find natural bioactive anti-bacterial products, and to evaluate the synergistic activity of combinations of plants extracts.
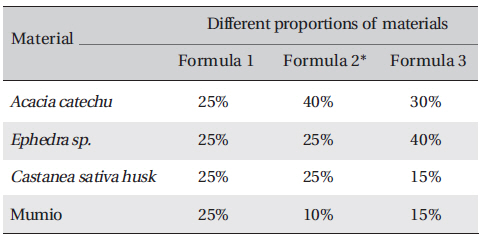
Processing of the plants performed in this study was the same as the traditional method used by the people in the Bakhtiari tribe. Aqueous extracts of the experimental materials were obtained and mixed in different weight ratios randomly, and anti-bacterial tests were carried out for all combinations. The most effective combination was then determined by comparing the results for the zone of inhibition. The experimental materials included: Mimosa catechu /
[Table. 1] Test materials formula in different weight ratios (W/W)
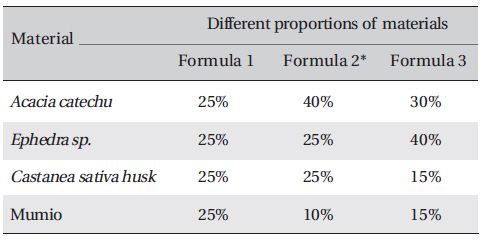
Test materials formula in different weight ratios (W/W)
Sample of all formula were prepared. Crude extracts were weighed and dissolved in a known volume of distilled water to obtain a final concentration for each of three formulae (Table 1) All formula was repeated three times. A variety of common bacteria were tested:
The density of bacteria was adjusted to 0.5 McFarland standards (1.5 x 108 CFU/ml) by adding sterile distilled water. McFarland standards were used as references to adjust the turbidity of the microbial suspension so that the number of microorganisms would be within a given range [11]. For the preparation of the 0.5 McFarland standard, 0.05 ml of barium chloride (BaCl2) [1.17% w/v (mass concentration) BaCl2.2H2O] was added to 9.95 ml of 0.18M H2SO4 (1.0% w/v) with constant stirring. The McFarland standard tube was tightly sealed to prevent loss by evaporation and was stored for up to 6 months. The test and the standard samples were compared against a white background with a contrasting black line (Andrews, 2001). Well-isolated colonies were transferred to tubes containing sterile distilled water to obtain microbial suspensions having turbidities close to that McFarland 0.5 standard (1.5 x 108 CFU/ml).
The bacterial isolates were sub-cultured on ready-made Nutrient agar (MP001) which was obtained from Hi Media Laboratory Pvt. Limited, (Mumbai, India). Nutrient agar plates were inoculated with 100 ㎕ of standardized inoculum (1.5 x 108 CFU/ml) of each selected bacterium (in triplicates); which was spread with sterile swabs. Wells or cups of 8 mm in size were made with a sterile borer into the agar plates containing the bacterial inoculum. A volume of the plant extract, 250 ㎕ was poured into a well of the inoculated plate. The Petri dishes thus prepared were left at room temperature for ten min to allow for the diffusion of the extract into the agar [12]. After incubation for 24 h at 37℃ , the plates were observed. Anti-bacterial activity was indicated by an inhibition zone surrounding the well (including the well diameter) containing the plant extract. The zone of inhibition was measured and expressed in millimeters. Anti-bacterial activity was recorded if the zone of inhibition was greater than 8 mm [13]. The interpretation of the anti-bacterial activity results was done according to the diameter of zone of inhibition as follows: < 9 mm zone was considered as inactive, 9-12 mm as partially active, 13-18 mm as active, and > 18 mm as very active [14]. The means and standard deviations of the diameters of inhibition zones were calculated.
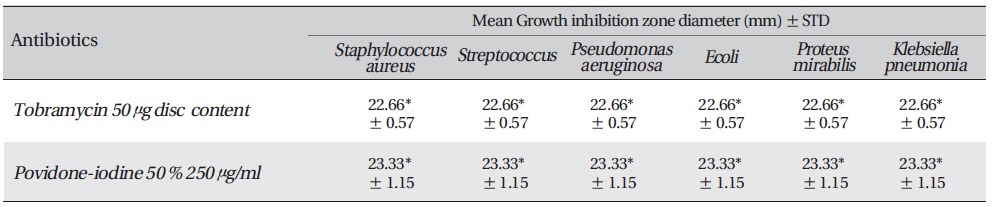
The standard anti-bacterial agents were Povidone-iodine, Tobramycin 50 μg disc. Anti-biogram was done by the Kirby-Bauer method with antibiotic discs of tobramycin (50mcg) or Povidone-iodine. The results were interpreted per Clinical and Laboratory Standards Institute (CLSI) 2005 recommendations.
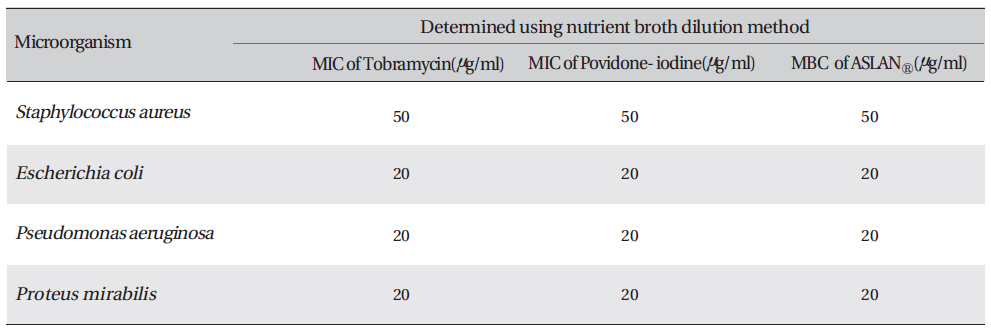
Petri dishes containing nutrient agar medium were seeded with 24-h cultured bacterial strains. Wells were made on the agar surface with an 8 mm cork borer. Aqueous plant extracts, 250 ㎕, were poured into the well by using sterile syringes. The plates were then incubated at 37℃ for 24 h. The anti-bacterial activity was assayed by measuring the diameter of the inhibition zone formed around the well (National Committee for Clinical Laboratory Standards Guidelines, 1993) [15]. The minimum inhibitory concentrations (MICs) and the minimum bactericidal concentrations (MBCs) for the aqueous extracts formula were proposed for six microorganisms [16]. In the macro-dilution broth method, two-fold serial dilutions of the proposed aqueous extract formula were prepared in a sterile nutrient agar broth to achieve a decreasing concentration ranging from 1200 μg/mL to 37.5 μg/mL. Each dilution was seeded with 50 ㎕ of the standardized bacterial inoculum (1.5 x 108CFU/ml). The inoculated culture tubes were incubated at 37℃ for 18 to 24 h. A set of tubes containing only seeded broth (i.e, without plant extract) was kept as a control. The lowest concentration that did not permit any visible growth when compared with the control was considered to be the MIC. The herbal aqueous extract is dark brown in color, so a visual observation of growth versus inhibition was not possible. Thus, mixture solution was swabbed in suitable media. After incubation at 37℃ for 24 h, the plates were examined for growth of bacteria to determine the minimum concentration of the extract required to kill 99.9% of bacterial isolates.
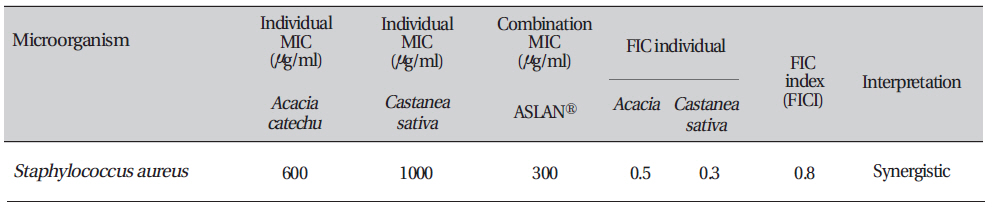
The fractional inhibitory concentration for each compound was calculated as follows: FICA (fractional inhibitory concentration) = (MIC of compound A in the presence of compound B)/(MIC of compound "A" alone). Similarly, the FIC for compound B was calculated. The FIC index (FICI) was calculated as FICI = [FICA + FICB]. Synergy was defined by FICI values ≤ 0.5, antagonism by FICI values > 4.0, and no interaction by FICI values from 0.5 to 4.0.
Data were evaluated using the IBM SPSS software program (version 19; IBM SPSS Inc., IL USA). Herbal extract and control groups were compared at the 95% confidence interval. Results were expressed as means ± standard deviations. Differences between the control group and the herbal extract groups were the criteria for the anti-bacterial activities. The
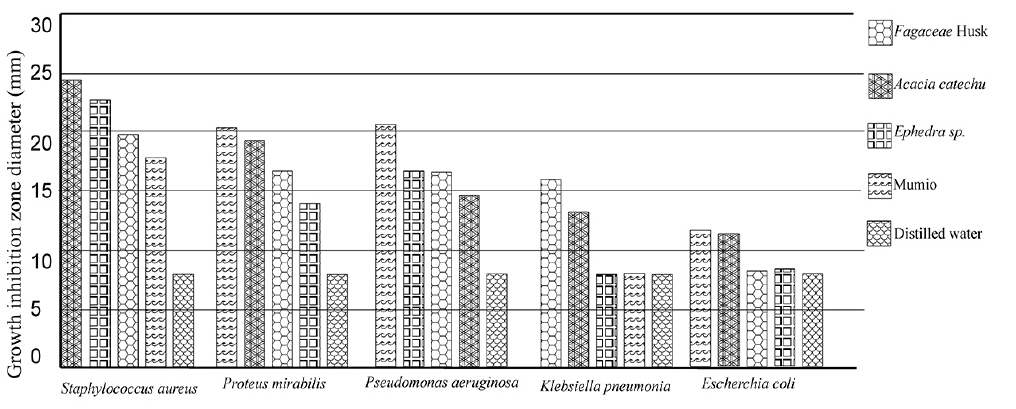
The results of the anti-bacterial screening as the zone of inhabitation obtained for
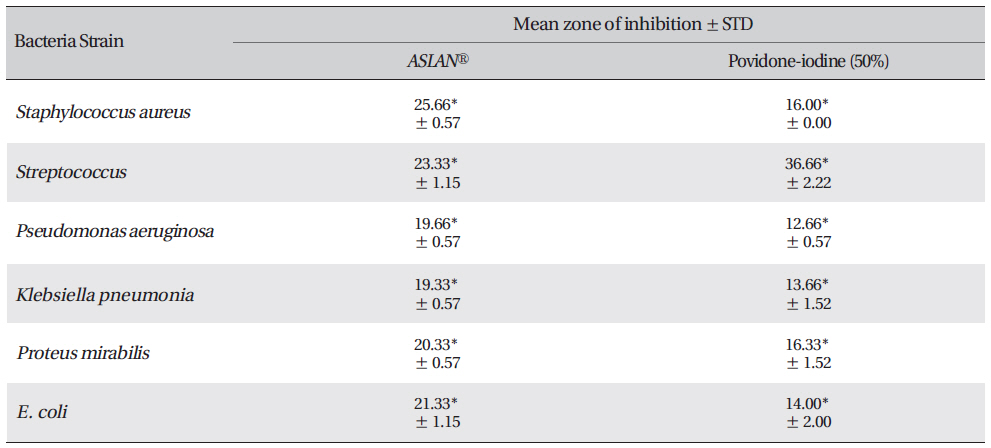
The inhibition zone diameters of the reference antibiotic and antiseptic for different pathogens are shown in Table 5. Of the three different combined formulas which were tested, the combined formula number 2 was selected as the most effective combination based on the anti-bacterial efficacy (Table 4) The MIC values for the combined of aqueous herbal extract (ASLANⓡ) against different pathogens are 300 μg/ml for
The fractional inhibitory concentration (FIC) is the lowest concentration of the antibiotic or the extracts combination permitting no visible growth of the test organisms on the plates [17]. The FIC value for each agent was calculated using the following formula:
FIC (Acacia catechu) = MIC of
The interactions between the
FIC Index = ΣFIC = FIC (
In this study we achieved a desirable synergistic effect with the combined formule. FIC index values < 1 were considered as synergetic and the degree of synergy increased as the value tended towards zero. Besides possessing these valuable synergistic effects, combinations of two or more compounds are desirable for the following reasons: (1) preventing or suppressing the emergence of resistant strains, (2) decreasing dose-related probable toxicity, (3) and attaining a broad spectrum of activity [18].
As "Prof. Jean-Marie Pelt" mentioned [19]: the therapeutic effect cannot be attributed to a single chemical constituent or a group of molecules. All the substances in the plant determine the activity of the plant by a synergistic, but sometimes an antagonistic effect. However, generation of the effect requires a substantial amount of a single ingredient, in other words, a higher concentration than normally found in the plant. The occurrence of side effects also increases with higher doses of the active ingredient. In theory, a plant extract with a low amount of the 'active ingredient' would be expected to have few side effects, but also little pharmaceutical effect. However, herbs have been observed to be effective in a variety of conditions while at the same time they appear to be associated with fewer side effects. The pharmaceutical activity of herbal compositions may be attributable to the natural presence of multiple biologically-active compounds that interact in synergy to create an effect that is greater than that which occurs with a single chemical component. As no single component is present in a large quantity, side effects are lessened. Antiseptics are often used for the treatment of skin and mucous membrane wounds before invasive procedures, so their effectiveness must be relatively fast. This herbal antiseptic is suitable for treatment of wound, mucosal infections and deep chronic ulcers, in order to achieve fast effectiveness goal, more research is needed.
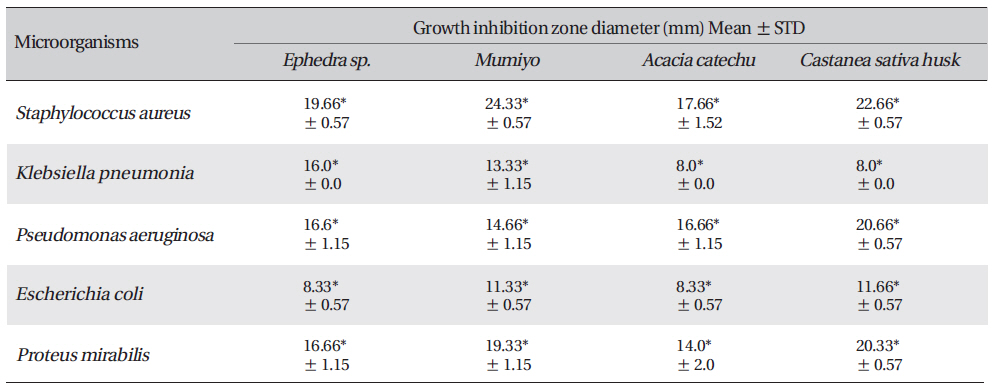
Zone of inhibition recorded with Acacia catechu (L.F.) Willd, Castanea sativa, Ephedra sinica stapf and shilajita mumiyo extracts against different pathogens
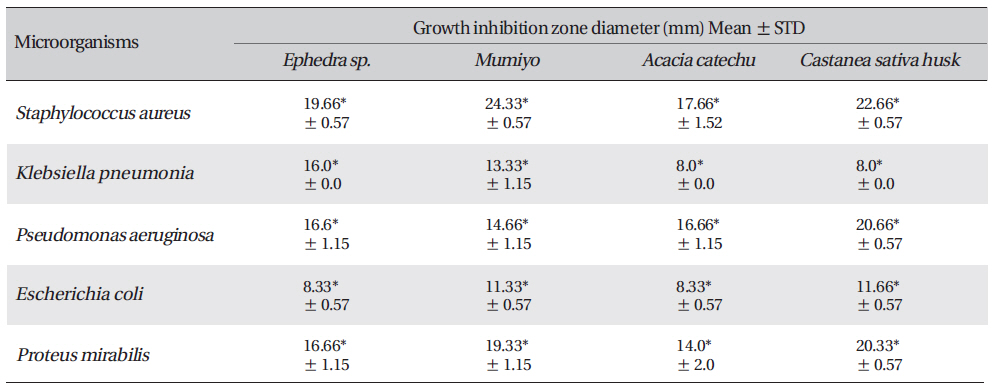
[Table. 3] Growth-inhibition-zone diameters of six bacterial species
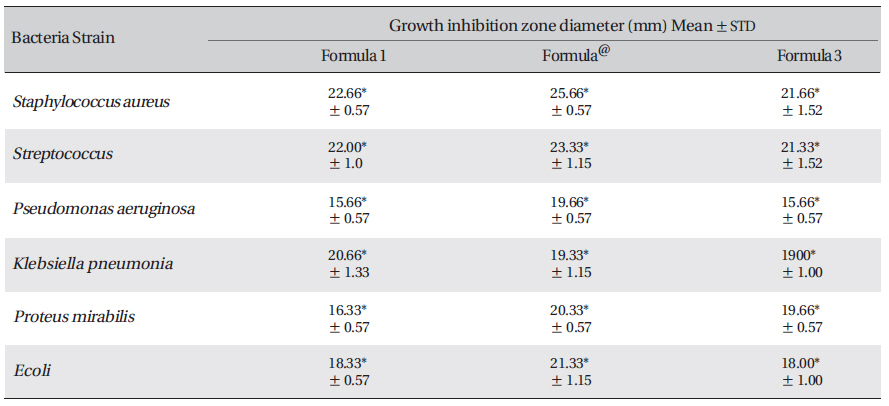
Growth-inhibition-zone diameters of six bacterial species
The results reveal the presence of antibacterial activity of
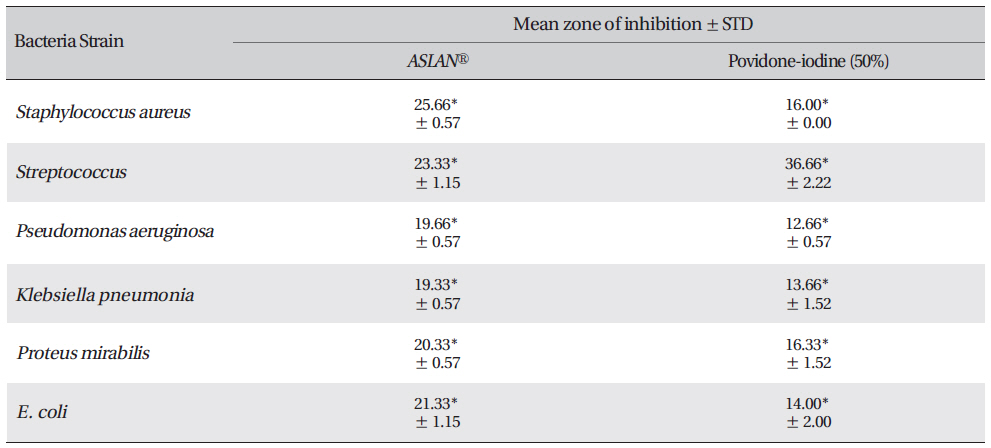
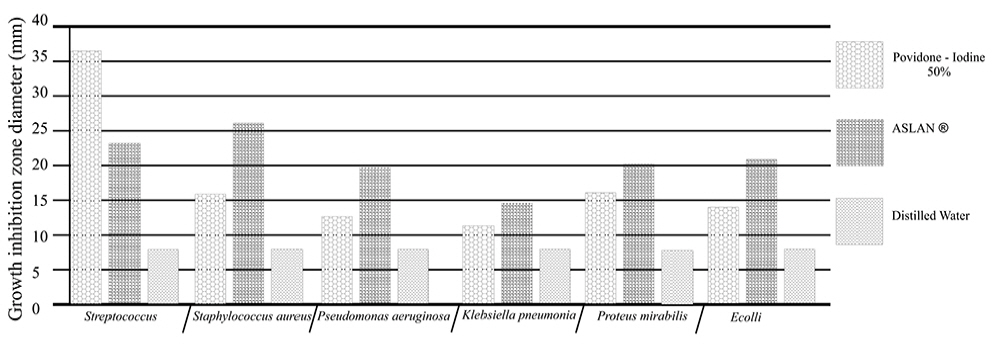
Mean zone of inhibition recorded with test material (ASLANⓡ) against different pathogens compared with that for Povidoneiodine.
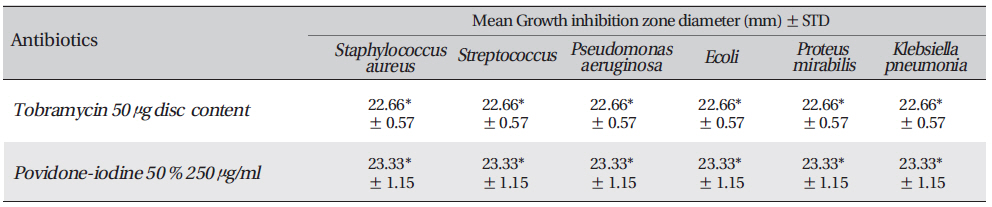
Growth inhibition zone diameter of the reference antibiotic and antiseptic against different bacteria
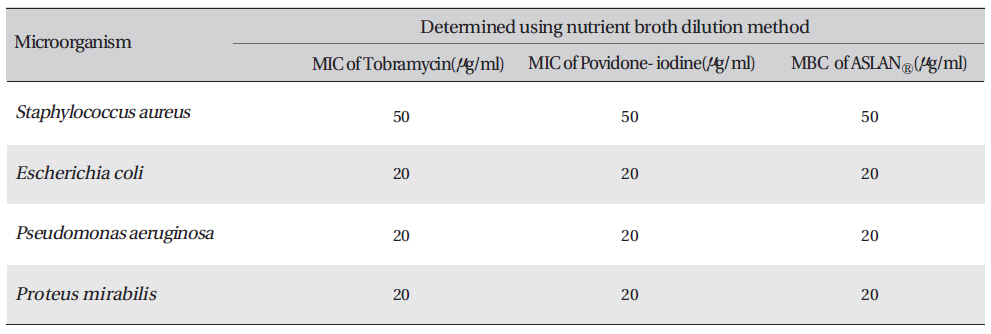
Determination of the minimum inhibitory concentration (MIC) and the minimum bactericidal concentration (MBC) of proposed aqueous extracts against four microorganisms
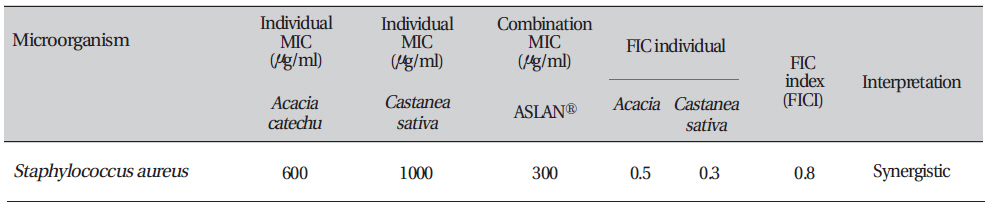
Combination testing of the aqueous extract of Aslanⓡ with individual ingredients (Acacia & Castanea sativa husk) against Staphylococcus aureus