The soil of regions with high salt concentrations support distinctive vegetation, and lower numbers of species are found in salt fields than in regions with either low or no salt concentration. Salinity is a major environmental stress and is a substantial constraint to crop production. Increased salinization of arable land is expected to have devastating global effects, resulting in 30% land loss within next 25 years and up to 50% by the middle of the 21st century (Wang et al. 2003). Therefore, new approaches are necessary to cope with these problems. One option is the use of more halophytic crop species, which can tolerate high levels of soil salinity.
Plants growing well under saline conditions necessarily have a greater salt tolerance, and this characteristic has influenced the ecological distribution of various plants (Flowers and Colmer 2008). By accumulating inorganic ions, halophytes absorb water by maintaining a high osmotic potential (Bradley and Morris 1991, Volkmar et al. 1998, Li et al. 2010). Betaine and proline are compatible solutes that accumulate in response to osmotic stress, and the accumulation of these osmolytes represents an important adaptive response to salt and drought
stress (Rhodes and Hanson 1993, Di Martino et al. 2003, Moghaieb et al. 2004). Halophytes are being used not only as food crops and fodder, but also as ground cover to protect against soil erosion. Through the root development, soil porosity increases, and the physical condition of the soil is subsequently improved over time (Park et al. 1983, Rozema and Flowers 2008).
Chenopodiaceae are comprised of ~1,500 species in ~100 genera and are distributed worldwide. They are known to be a representative plant community with unique ecological habitats, such as deserts and salt marshes (Heywood 1993, Akhani et al. 1997). In Korea, 15 species (7 genera) have been reported, and are mostly distributed in coastal sand dunes and salt marshes (Lee 1988, Yang 1999).
From previous studies of genus
It is therefore essential to study plants with resistances to environmental stresses in order to create crops that can withstand stress conditions such as drought and increased salinity. Some plant species may possess unique physiological characteristics which critically influence environmental adaptation. It is therefore very necessary to identify these characteristics in order to investigate the environmental adaption of particular species (Choo and Albert 1997, Chimenti et al. 2002).
In this study, the changes of seasonal growth and plant density of
The study site was located in Naeyang-ri, jido-eup, Sinaan- gun, Jeollanam-do, Korea (Fig. 1). The annual mean temperature and precipitation in the study area were 13.8℃ and 1,125 mm, respectively. This study site is an abandoned farm where a community of
>
Soil sampling and measurement of chemical characteristics
Soil samples 15-20 cm below the surface of the plant were also collected to analyze the soil that the plants inhabit.
The collected soil samples were air dried. Soil samples (5 g) were added to distilled water (25 mL) and were shaken for 1 h. After the soil solution was filtered through filter paper (Whatman No. 40, 110 mm), soil pH, total ionic contents (calculated as NaCl equivalents) and chloride contents were measured using a pH meter (Orion US/710; Thermo Orion, Beverly, MA, USA), electronic conductivity meter (Mettler Check Mate 90; Mettler-Toledo Inc., Columbus, OH, USA) and chloride titrator (Titrators DL 50; Mettler Toledo Inc.).
Exchangeable cations (Na+, K+, Ca2+, Mg2+) of soil solution extracted by 1 N ammonium acetate (CH3COONH4), were quantified using an inductively coupled plasma atomic emission spectroscopy (ICP-AES, Optima 7300 DV; Perkin Elmer, Waltham, MA, USA).
>
Plant sampling and plant water content
Leaves of
>
Measurement of inorganic ions
The dried plant material was ground to a homogeneous powder and was extracted with 95℃ distilled water for 1 h, after which the sample was filtered with a GF/C filter (pore size 1.2 μm). Inorganic cations (K+, Mg2+, Ca2+, Na+) were determined by ICP-AES. The chloride content was measured using a chloride titrator (Titrators DL 50; Mettler Toledo Inc.).
>
Measurement of water-soluble carbohydrates, osmolality and amino acids
Total water-soluble carbohydrates of the plants were assayed using phenol-sulfuric acid method (Chaplin and Kennedy 1994). 20 μL of plant extract was mixed with 580 μL of distilled water, 400 μL of 5% phenol and 400 μL sulphuric acid, The solution was allowed to stand for 10 min before being shaken vigorously. Total carbohydrates were quantified through determining the absorbance at 490 nm using a spectrophotometer (UV mini 1240; Shimadzu, Kyoto, Japan) after a further 30 min. Glucese (2-40 μg in 200 μL) was used as standard solution.
Osmolality was measured by cryoscopy using an osmometer (Micro-Osmometer 3MO; Advanced Instruments, Needham Heights, MA, USA). Free amino acids were quantified using an amino acid analyzer (L-8900; Hitachi, Tokyo, Japan).
Data was analyzed by analysis of variance (ANOVA) using SPSS ver. 19.0 (SPSS Inc., Chicago, IL, USA). Graphs show means with standard error (s.e.). A Duncan’s multiple range test was carried out to determine significant differences (
The investigation area has a low water content, being dependent on rainfall alone, even though it is composed of clay.
>
Chemical characteristics of soil
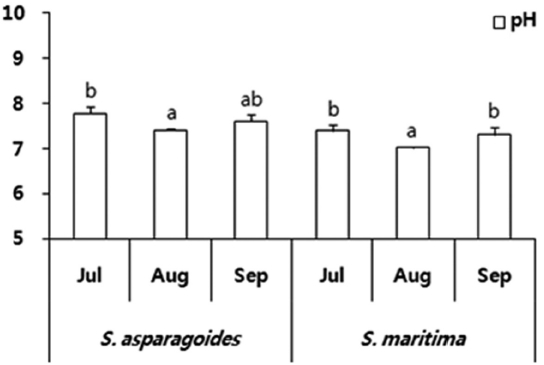
The soil was sampled once a month from July to September in 2011. Generally, the saline soil of the sample site showed pH values of 8.26-9.03 (Kim et al. 2006). However, the soil of investigation areas of
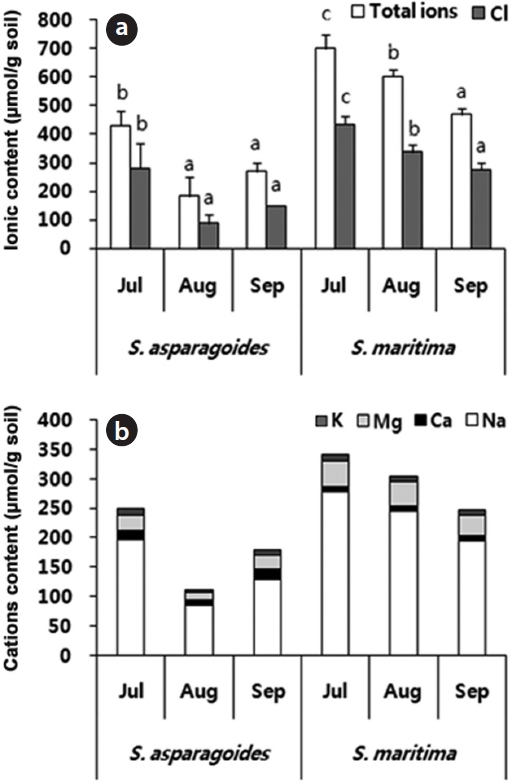
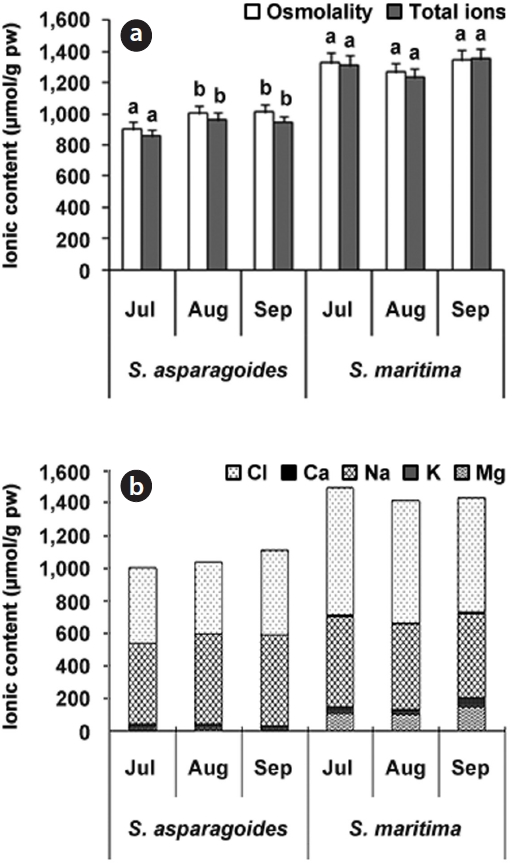
The total ion and Cl- content of soils in which both species appeared showed a gradual decrease throughout the survey period. The soil of
The soil of
total ion concentration of 468.5 μmol/g soil and Cl- of 275.5 μmol/g soil in September (Fig. 3). The reduction in the total ion content of soil was ascertained by the reduction in exchangeable Na+and Cl- content. It seems that this study area was desalinated by rainfall in August and plant uptake. As soil desalinization progressed, soil was classified as first saline-sodic soil, the next saline soil and then normal soil (Lee et al. 2003). The exchangeable soil Na+ contents were 86.14-197.67 μmol/g soil for
>
Growth characteristics of plant
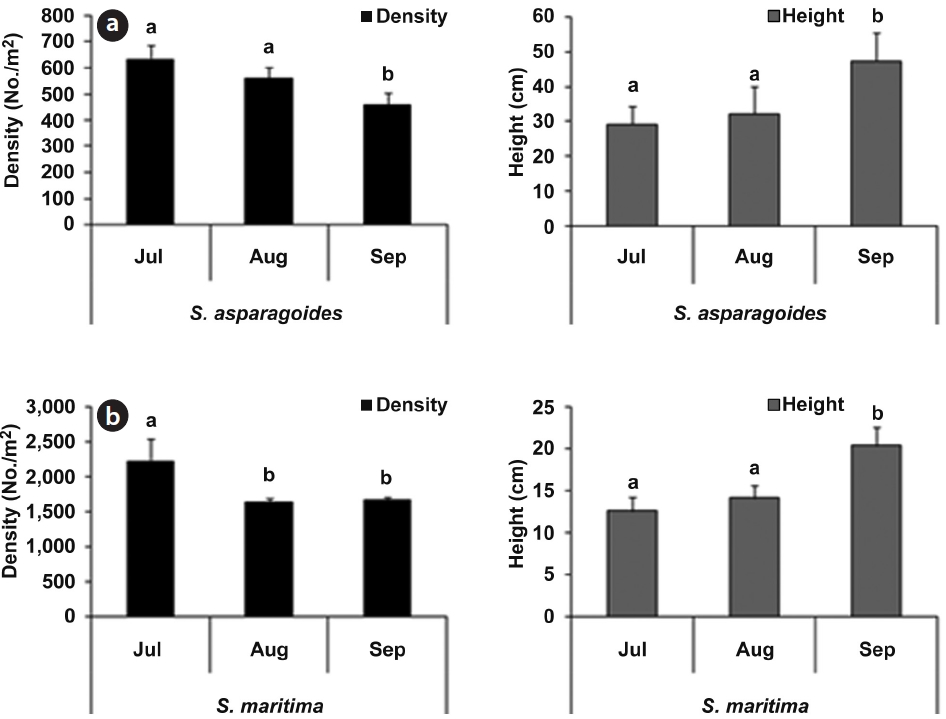
As part of the growth characteristics of
The community of
The plant density of
>
Physiological characteristics of plant
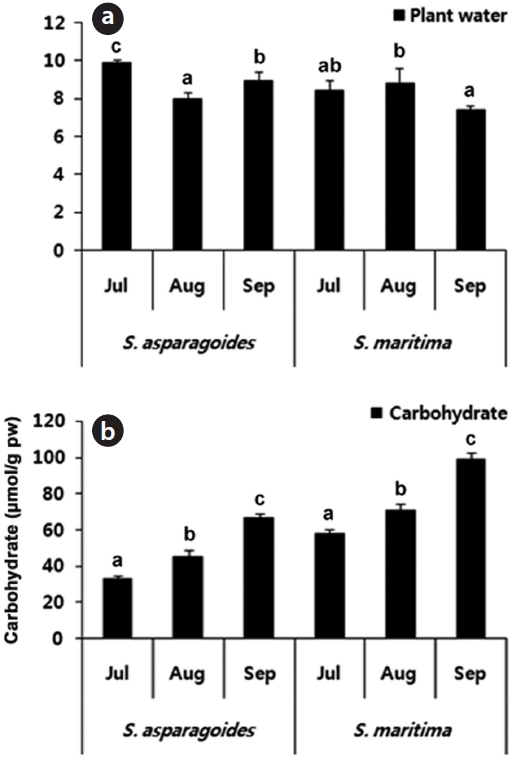
Leaves of
By maintaining a high ratio of water and tissue succulence, some chenopodiaceae and halopytes inhabiting in
saline habitats avoid ion toxicity by diluting accumulated Na+ and Cl-.
The osmolality of
Both plant species contain a small amount of soluble Ca2+ ions in their leaves. Generally, plants absorb Ca2+ ions in greater concentrations than those required for the maintenance of basic life activities, but the Ca2+ ion concentration in cytosol is extremely low because excessive internal Ca2+ ions can be harmful in certain conditions (Marschner 1995). Usually Ca2+ ions extracted by hot water originate from the vacuole, cytoplasm and organelles, and some fractions extracted by HNO3 or HCl are known to contain oxalate bound Ca2+ ions (Kinzel 1989). Chenopodiaceous plants accumulated most incoming Ca2+ ions in the form of Ca-oxalate within vacuoles, therefore free Ca2+ ion exist in very small quantities in leaves of Cheno-
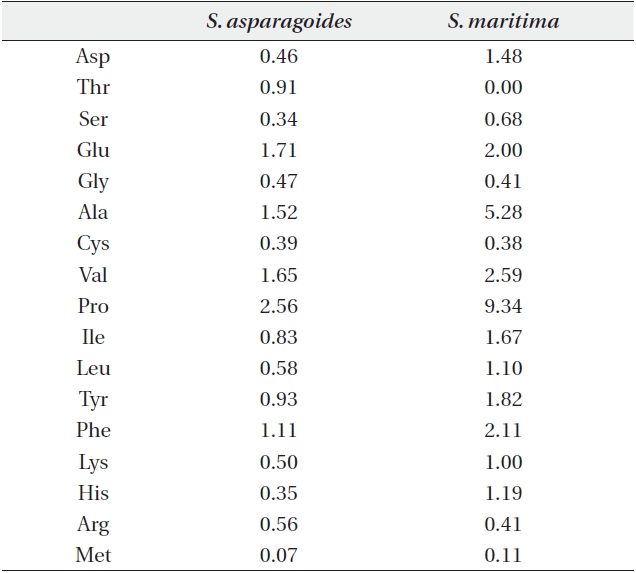
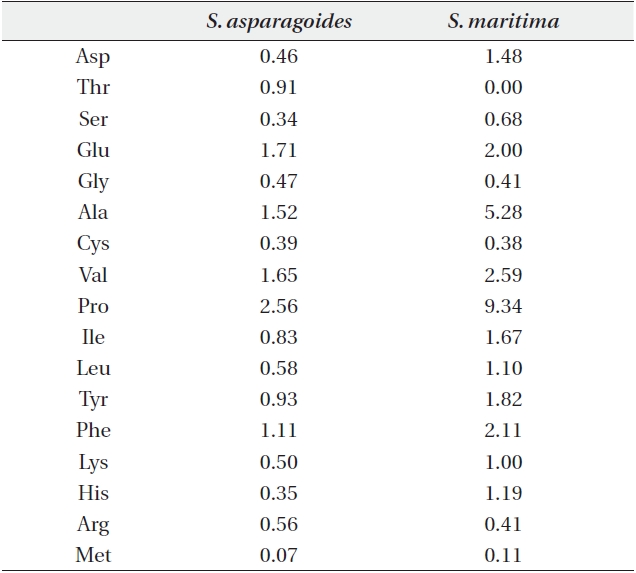
Free amino acid content (μmol/g plant water) in leaves of Suaeda asparagoides and S. maritima
podiaceous plant species (Kinzel 1989, Choo and Song 1998).
The content of free amino acids in the leaves of
The accumulation of proline, known as one of compatible solutes, is a common metabolic response of higher plants to water deficits, salinity stress and cold stress, and proline accumulation may play a major role in osmotic adjustment. Proline influences protects against biologically unfavorable consequences of dehydration (Binzel et al. 1987, Voetberg and Sharp 1991, Rhodes and Hanson 1993).
By identifying the low content of proline in the investigated
The habitats of
Under the saline conditions observed within the study area,
These results suggest that