Ecological health is a multidimensional concept that cannot be easily measured or monitored. Nevertheless, the composition and structure of soft-bottom macrofaunal communities are successfully used to characterize environmental conditions and to estimate the extent of environmental impact, as indicators for ecological health (Nyenje et al. 2010). Lagoons have four major categories of sustainability: the production of food and water; the control of climate and disease; the cycling of nutrients and pollination of crops; and cultural benefits, such as providing spiritual and recreational grounds for mankind (Tlig-Zouari et al. 2009). Ghana is involved in the Guinea Current Large Marine Ecosystems initiative. The fisheries of almost all the lagoons and estuaries in Ghana are artisanal (large subsistence) but play an important role in the socio-economy of coastal inhabitants in terms of employment and the provision of fish protein. In addition to providing habitats for fish, lagoons are important staging, feeding and roosting areas for migratory birds. Currently, lagoons and estuaries of this region are under stress from anthropogenic interferences such as changing land use, destruction of fringing mangroves, discharges of domestic and industrial wastes and overfishing (French et al. 1995, Armah et al. 2010). The most severe direct results of such pressures on estuaries and lagoons in Ghana are the reductions in freshwater inflow and the deterioration of water quality. Additionally, climate change poses a potentially serious future threat to estuaries and lagoons. Even with moderate rises in sea levels these low-lying ecosystems are at risk of inundation and further erosion (French et al. 1995).
Deterioration of the ecological health of lagoons leads to dissolved oxygen (DO) deficits, aquatic toxicity, variation in organism composition, disappearance of benthic organisms, turbidity and eutrophication-induced odours, reductions in fish size, and fish mortality (Specchiulli et al. 2009, Armah et al. 2010).
Several methods and indices have been used to assess the impact of increasing pollution on the ecological health of lagoons. The Reish Method uses the distribution pattern of pollution-tolerant polychaetes, particularly
This study focuses on two pollution sensitive coastal lagoons: a closed lagoon (Fosu) and an open lagoon (Benya), both of which have experienced anthropogenic pressures, although the magnitude and frequency of pollution is not uniform throughout the two. The Fosu Lagoon is considered to be highly polluted (Armah et al. 2010) due to the disposal of waste, water withdrawal for irrigation, reduction in size of the popular black chin tilapia in recent years, and increasing severity of algal blooms (Gilbert et al. 2006, Darkwa and Smardon 2010). Discharge into the lagoon from a cluster of mechanical workshops a source of Iron (Fe), Magnesium (Mn), Cadmium (Cd), Zinc (Zn), and Nickel (Ni) in the Fosu Lagoon (Gilbert et al. 2006). Though there is no industrialization around the Benya Lagoon, its proximity to a fish market, which serves about 500 individuals weekly, creates the likelihood of waste and effluent disposal through the associated anthropogenic activity. A naturally eutrophic-mesotrophic environment is gradually shifting towards eutrophic-dystrophic conditions (Yankson and Kendall 2001). This situation threatens the sustainability of fisheries, and therefore suggests the need for an assessment of the ecological health status of the two lagoons. Consequently, the aim of this study is to determine the physicochemical parameters, and to characterize and quantify the macro-benthic fauna in sediments, in order to estimate pollution levels in these sensitive ecosystems.
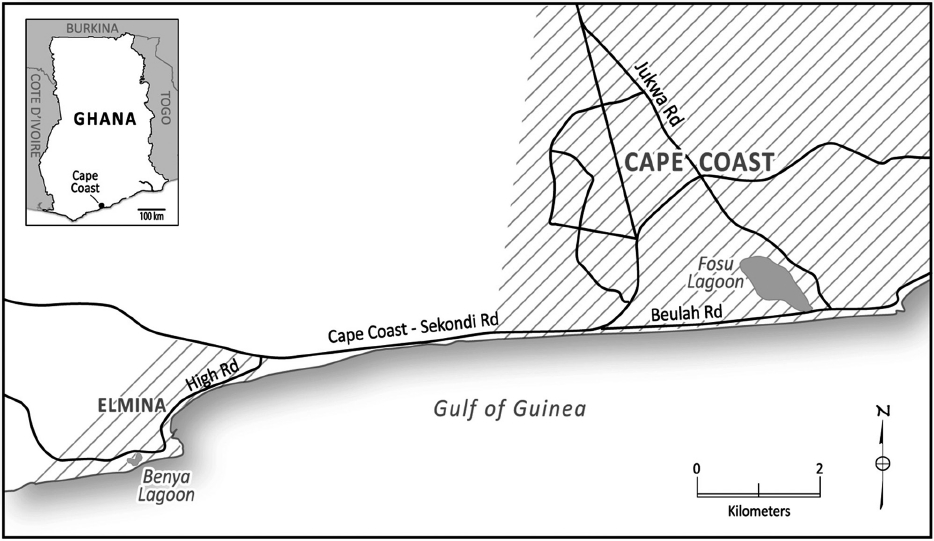
The Fosu Lagoon (5°6′17.87″ N, 1°15′18.68″W) is a closed coastal lagoon in Cape Coast, Ghana. It is a shallow brackish body of water separated from the Gulf of Guinea by a sand bar, which is frequently removed by heavy rainfall, or by the local people as part of a series of annual rituals. The temperature of the Fosu Lagoon ranges between 26-33℃. Apart from its significance to the traditional heritage of the people, it provides a livelihood for fishermen (Armah et al. 2010). The Benya Lagoon (5°05′ N and 1°22′ W) is an open Lagoon that is in contact with the sea throughout the year, and therefore, is under tidal influence. The temperature of the Benya Lagoon ranges between 24-32℃ and the salinity between 10-40 psu. The depth of the Fosu Lagoon ranges between 0.3 m and 0.8 m; and the depth of the Benya Lagoon ranges between 0.5 m and 2 m. Both lagoons are shown in Fig. 1.
Benthic sampling
This study provides a short-term picture of water quality (i.e., synoptic surveillance study) and is intended to describe the water quality at specific locations, as well as an opportunity to develop a sampling network and long term monitoring program. It is necessary to provide indications of whether more (or possibly fewer) samples are needed in order to gain knowledge of the water quality at various points throughout a water body, in the long term. Four main sites were each selected at the Fosu and Benya lagoons. These sites reflect different pollution sources, including settlement, industrial, and household sewage. The average distance between the observing sites and the pollution source was 25 m. Regarding the Fosu Lagoon, site 1 was located near the drainage; site 2 near the metropolitan hospital; site 3 in the middle of the lagoon; and site 4 near the mechanic workshops. For the Benya Lagoon, Site 1 was located under a bridge-outlet to the sea; Site 2 near human settlements; Site 3 in the middle of the lagoon and Site 4 (near the
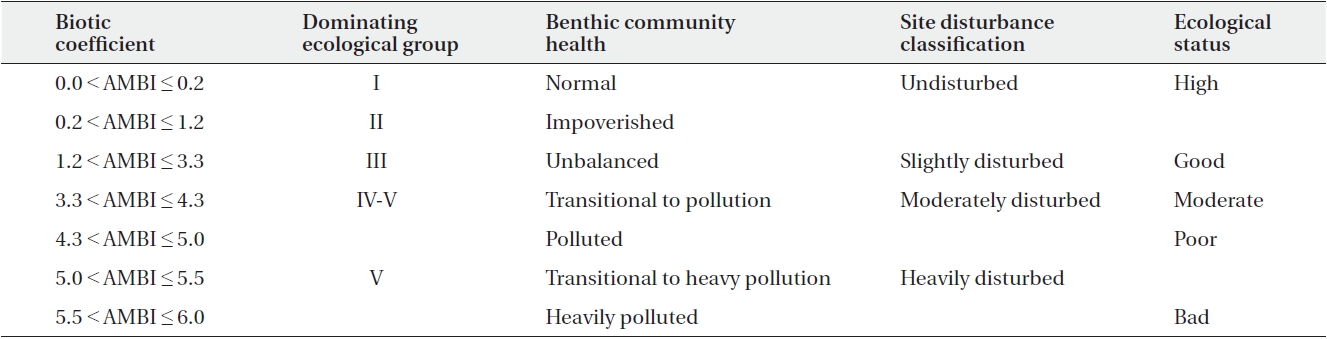
AMBI = {(0 × %GI) + (1.5 × %GII) + (3 × %GIII) + (4.5 × %GIV) + (6 × %GV)/100}
where % in the AMBI calculation represents total abundance. AMBI and multivariate-AMBI (M-AMBI) were calculated using AMBI ver. 5.0 software (Borja and Muxika 2005). GI-GV refer to groups I to V. M-AMBI involved the ordination of samples based on the values of AMBI, number of species and diversity, followed by a factor analysis to determine the distance of samples from “High” and “Bad” endpoints. For polluted conditions the M-AMBI value is 0, and for unpolluted conditions the M-AMBI value is 1. The classification boundaries used were the standard boundaries determined by inter-calibration of benthic ecological status assessment between states along the Gulf of Guinea. Table 1 shows the AMBI values and their respective equivalences.
Water chemistry
The water quality measurements for the Fosu Lagoon were made at the same time as benthic sample collection.
Determination of particle distribution and textural class
Sediment samples were collected, weighed, washed in brine, and first air-dried at 25℃ and subsequently, oven dried at 30℃ (Dyer 1986). For physical analysis, the dried sample was sieved through a series of Wentworth sieves of variable mesh sizes and retaining the fraction each time, the sieve sizes were 0.5 mm (500 μ), 0.25 mm, 0.125 mm, and 0.0625 mm. Each fraction was carefully weighed.
[Table 1.] Summary of the AMBI values and their equivalences (Borja et al. 2000)
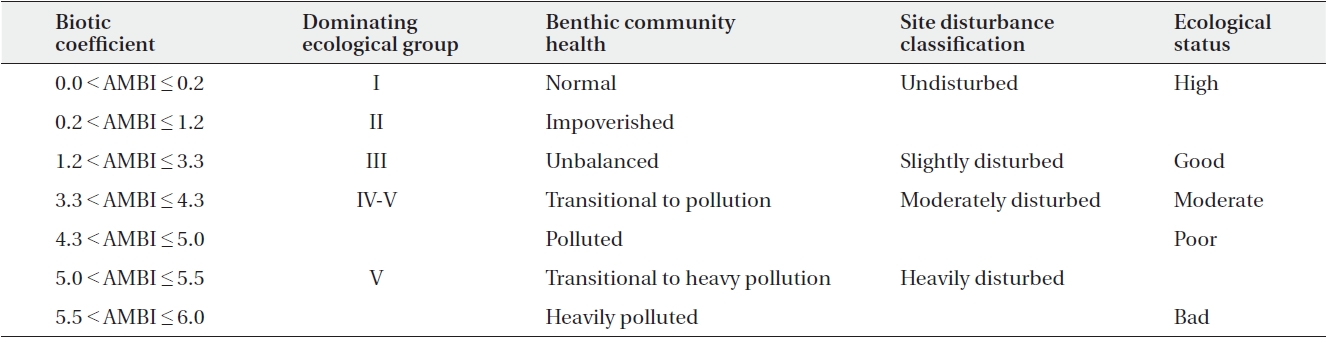
Summary of the AMBI values and their equivalences (Borja et al. 2000)
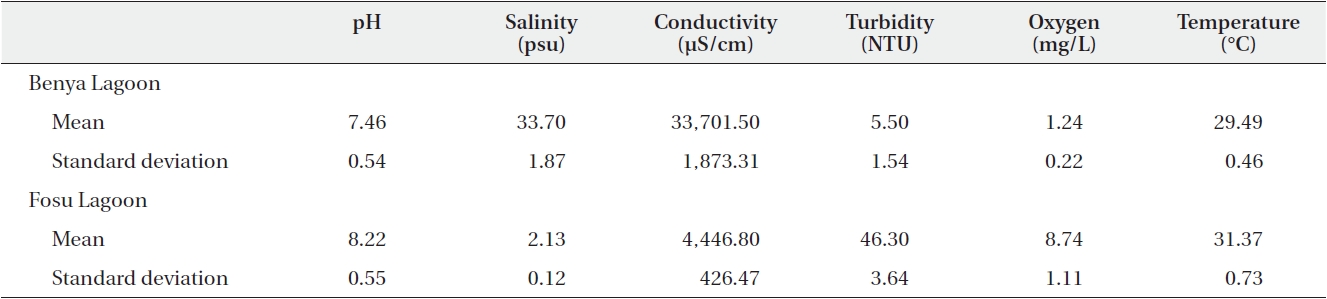
[Table 2.] Summary statistics of physicochemical parameters for the Benya and Fosu lagoons (N = 40)
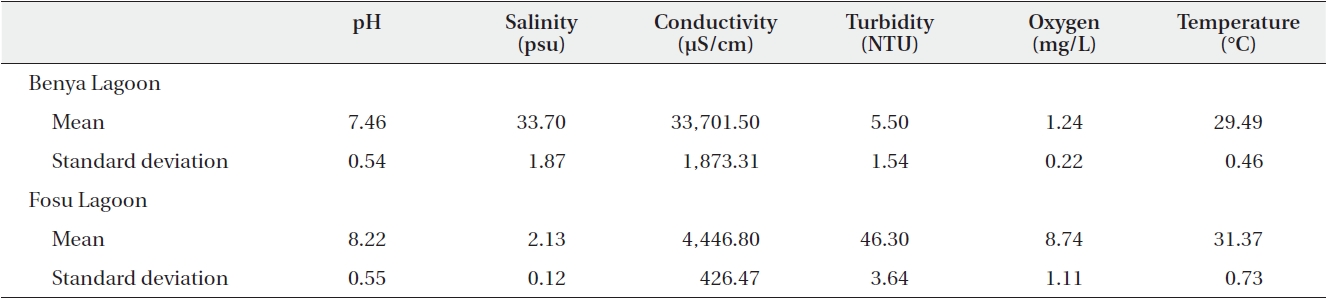
Summary statistics of physicochemical parameters for the Benya and Fosu lagoons (N = 40)
Descriptive statistics for the water quality data were performed with SPSS ver. 16.0 for windows (SPSS Inc., Chicago, IL, USA). The distributions were approximated to normal distribution based on Kolmogorov-Smirnov (K-S) tests. Relationships among water properties were tested using correlation analysis. Principal component analysis was employed to separate the physicochemical groupings inherent in the structure of the correlation matrix.
The pH values of the two lagoons (as presented on Table 2) were alkaline although the closed Fosu Lagoon had a higher pH than the open Benya Lagoon. There was a statistically significant difference between the mean pH for the two lagoons (
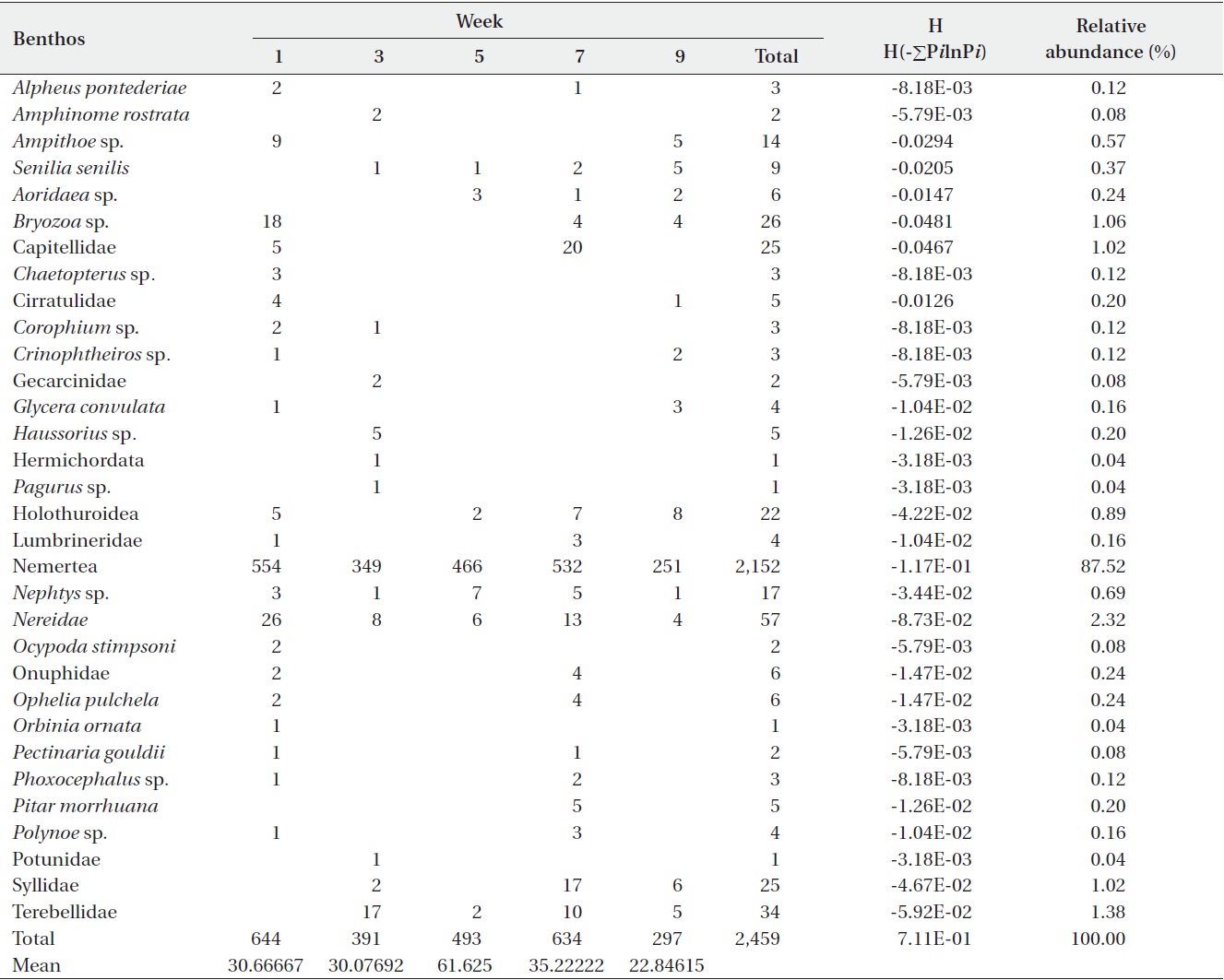
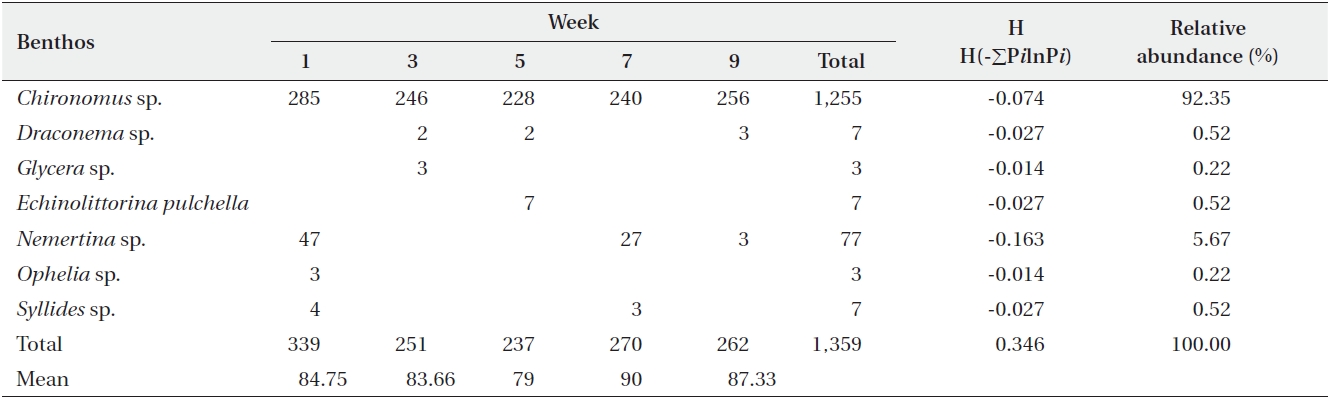
[Table 3.] Abundance of macro benthic fauna in sediments from the Benya Lagoon
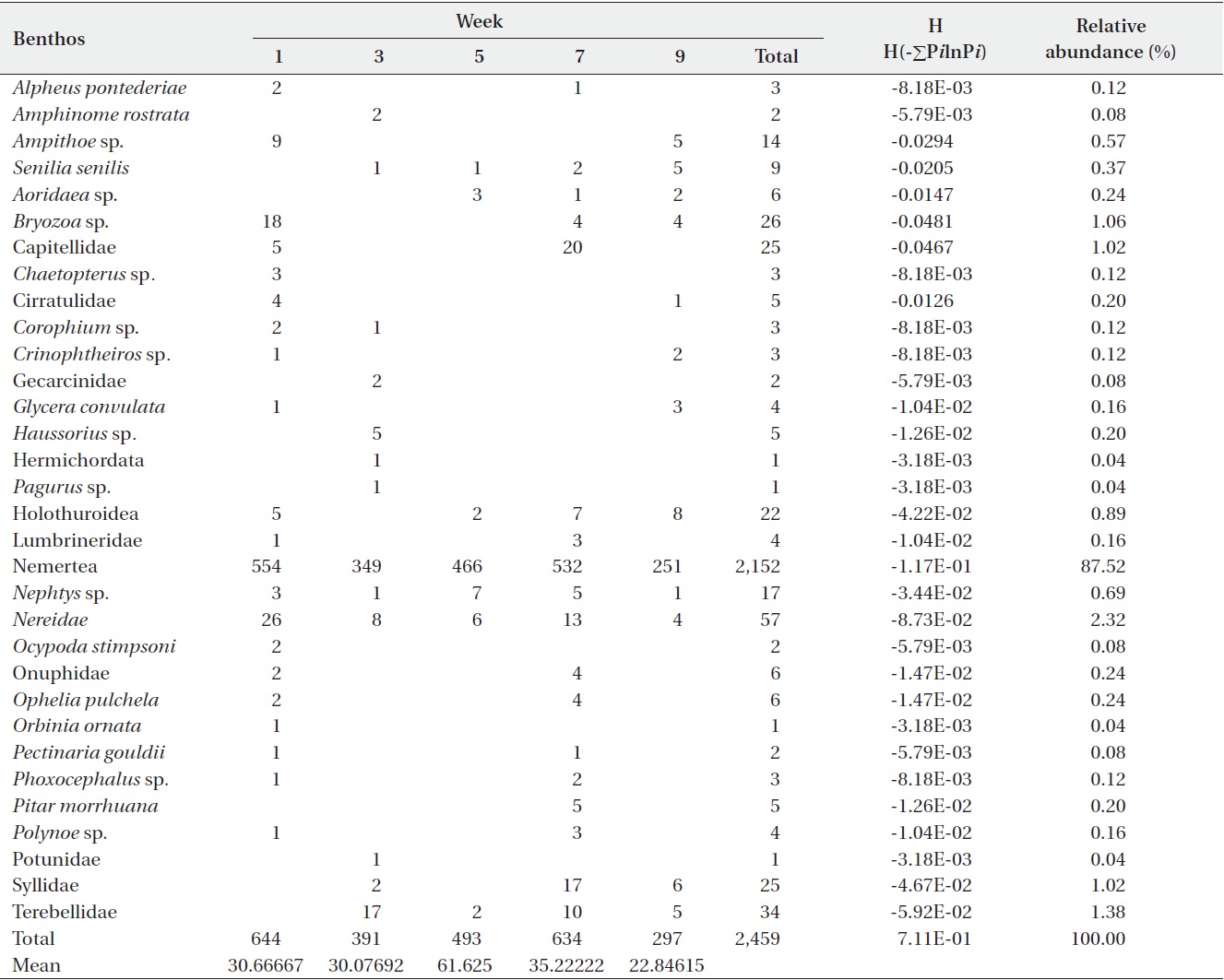
Abundance of macro benthic fauna in sediments from the Benya Lagoon
and Fosu lagoons, and in several cases, dramatic differences were observed. For instance, in the Fosu Lagoon, there were strong correlations between temperature and pH, conductivity and oxygen, and turbidity and oxygen, whereas a different set of associations were observed for the Benya Lagoon.
For the Benya Lagoon, it was observed that during low tides more diversified species were obtained than during high tides. About 2,459 individuals were distributed among 34 taxa in the Benya Lagoon. Only polychaeta and bivalves were widely distributed; however, gastropods, oligochaetes, phoronids and isopods were also important groups in terms of occurrence (Table 3). The diversity of species in the Benya Lagoon was recorded to be 7.11E-01 and the species with the most percentage abundance and numerical abundance was Nemertea (87.52% and 2,152, respectively).
>
Principal component analysis
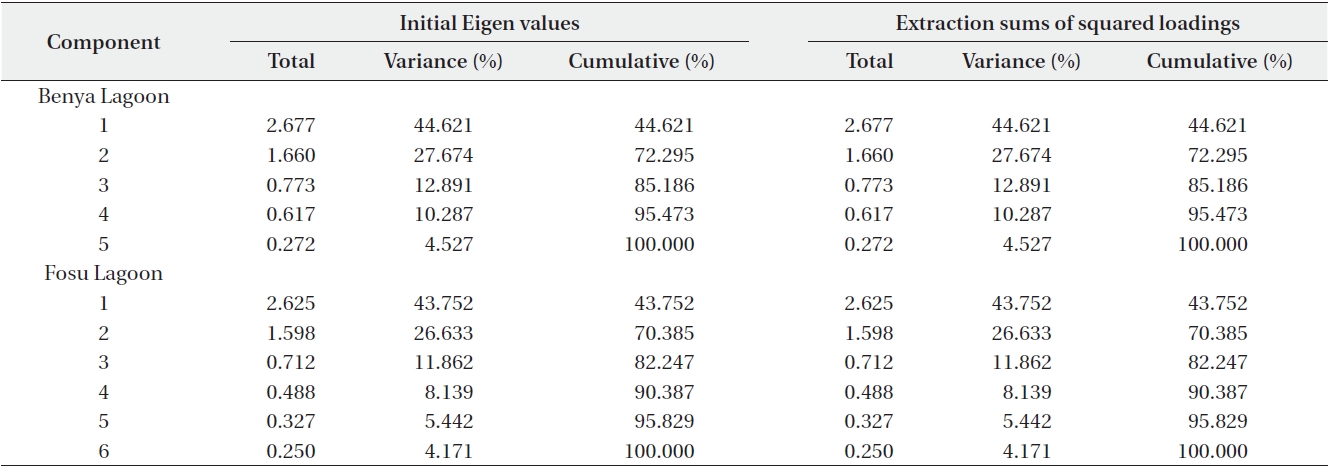
Two components each in the Benya and Fosu lagoons had Eigen values greater than 1. Cumulatively, these explain 72% and 70% of total variance in the Benya and Fosu lagoons data, respectively (Table 7). For the Benya Lagoon, salinity and conductivity showed strong positive loadings on factor 1 (natural hydrochemical properties).
[Table 4.] Abundance of macrobenthic fauna in sediments from the Fosu Lagoon
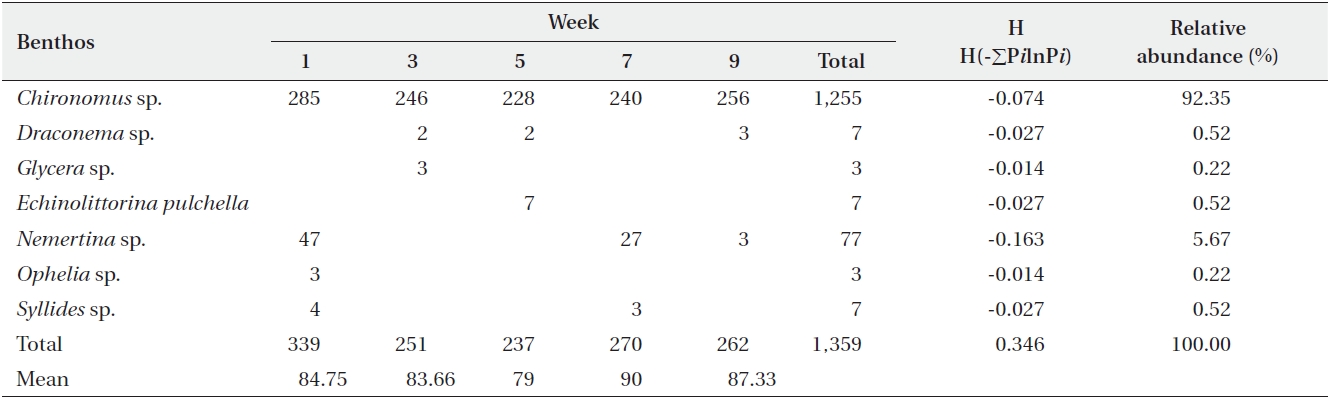
Abundance of macrobenthic fauna in sediments from the Fosu Lagoon
This could be attributed to the periodic flushing between the lagoon and the Atlantic Ocean. Also, oxygen showed strong positive loadings on factor 1 whereas temperature showed strong negative loadings on factor 1. This is understandable as flushing is also a process that is coupled with oxygen cycling. Turbidity exhibited strong positive loadings on factor 2 (land use) whereas pH showed strong negative factor loadings on factor 2. Turbidity of the Benya Lagoon is connected with the market activities close to the lagoon. For the Fosu Lagoon, conductivity, oxygen and temperature exhibited strong positive factor loadings on factor 1.
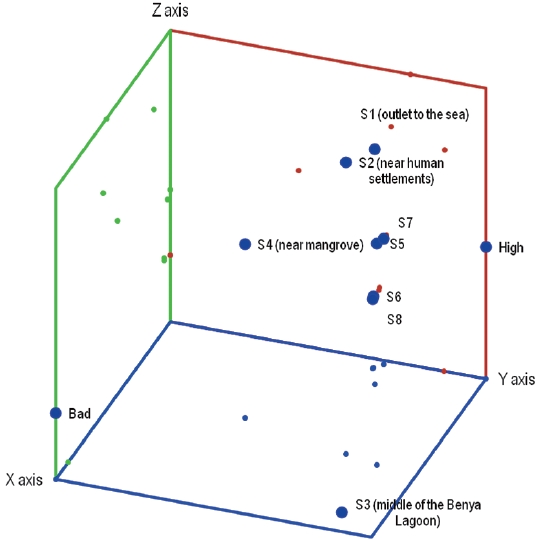
Benthic species near human settlements and sea outlets were similar, and indicated similar pollution levels (Fig. 2). The benthic species near the
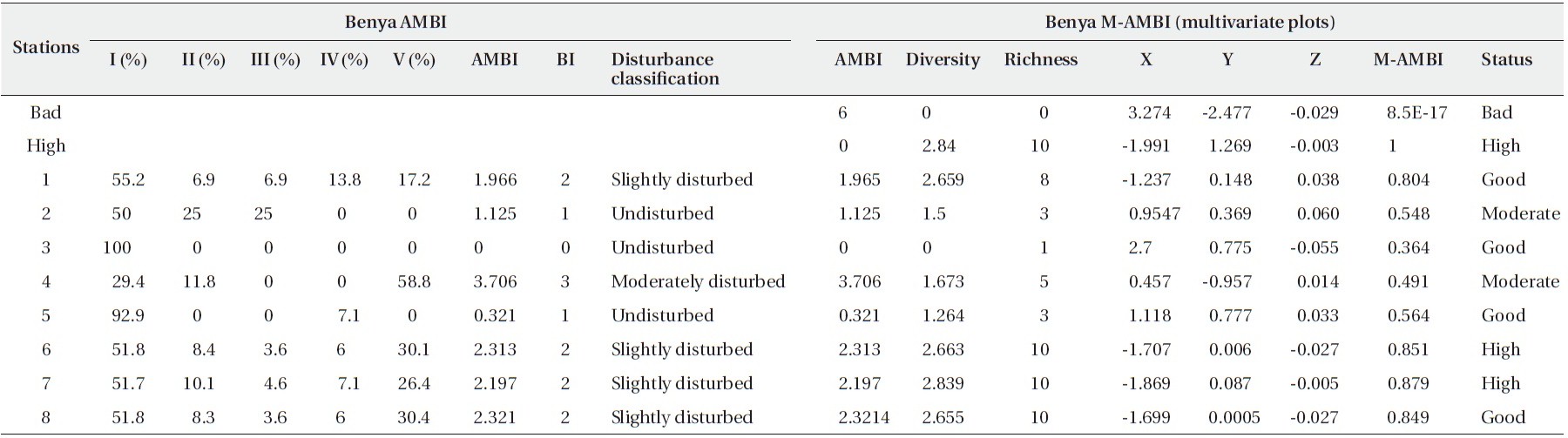
[Table 5.] Results of AMBI and M-AMBI for the Benya Lagoon
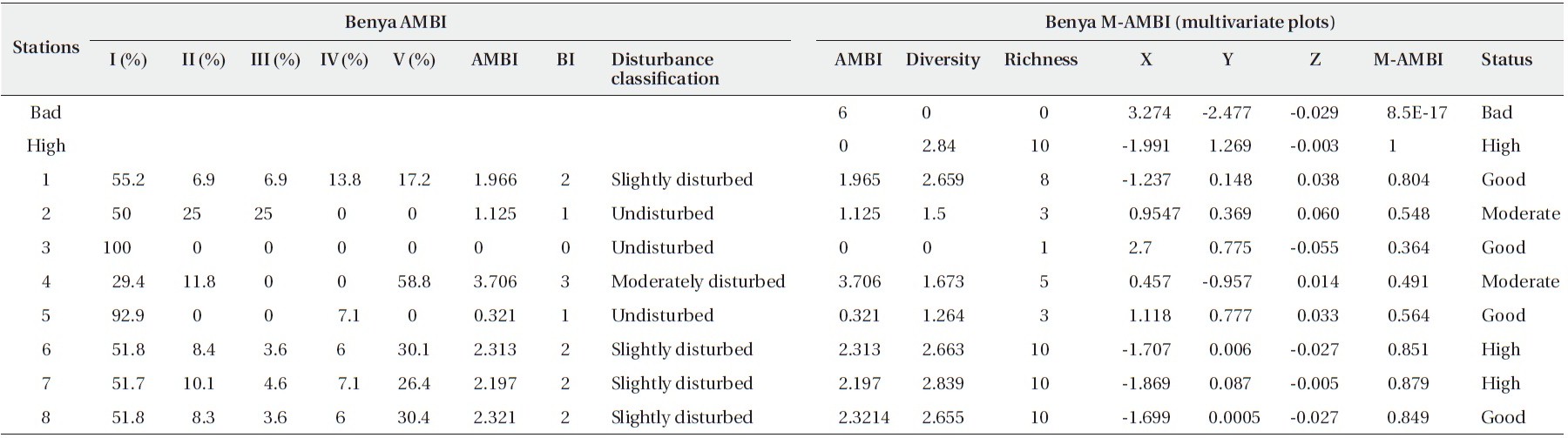
Results of AMBI and M-AMBI for the Benya Lagoon
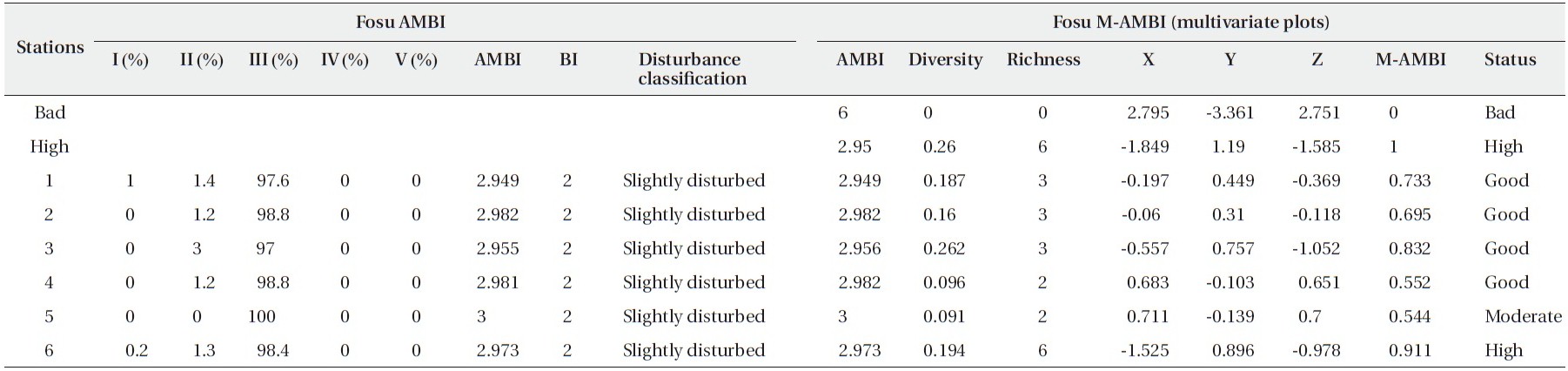
[Table 6.] Results of AMBI and M-AMBI for the Fosu Lagoon
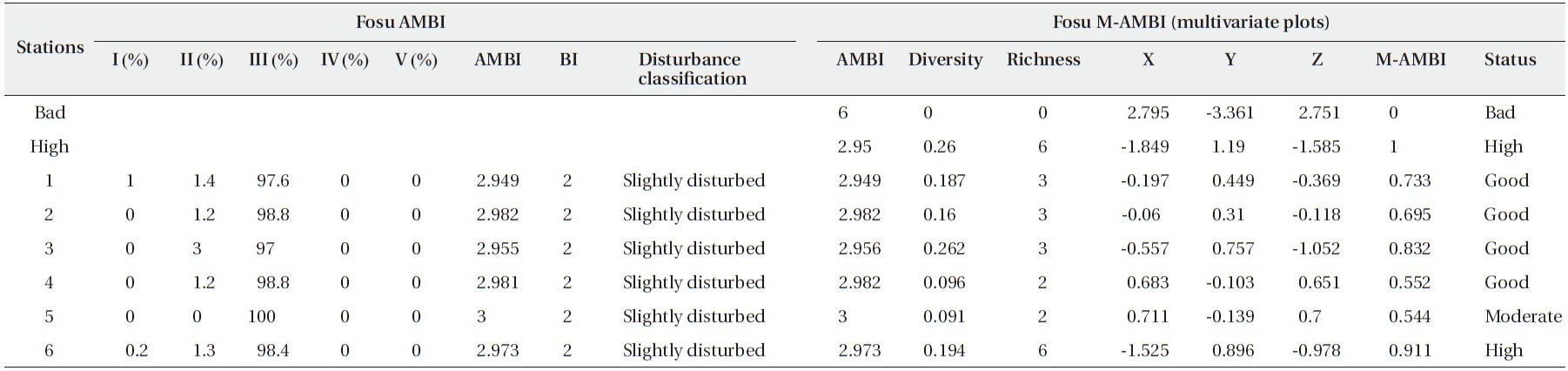
Results of AMBI and M-AMBI for the Fosu Lagoon
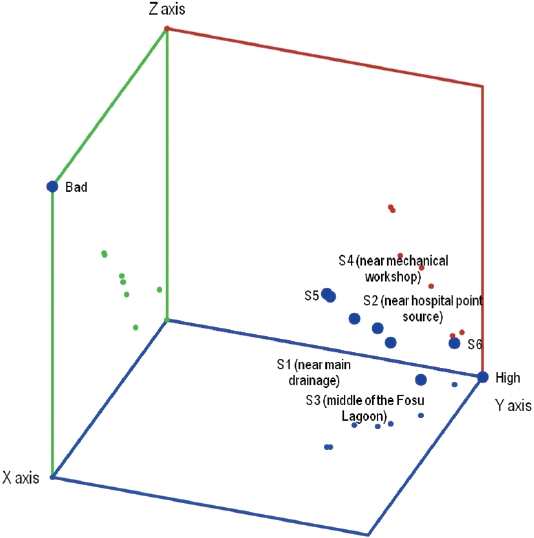
are high, S1, S5 and S8 are good in terms of benthic species diversity whereas S3 is poor (Table 5 and Fig. 2).
From Fig. 3, S1 to S4 are good whereas S5 and S6 are moderate and high in terms of pollution, respectively. Based on the clustering of the sites, the benthic species inindicate similar pollution levels.
The Fosu Lagoon was characterized by slightly disturbed conditions and macrobenthic assemblages that showed some indications of deterioration. These could be
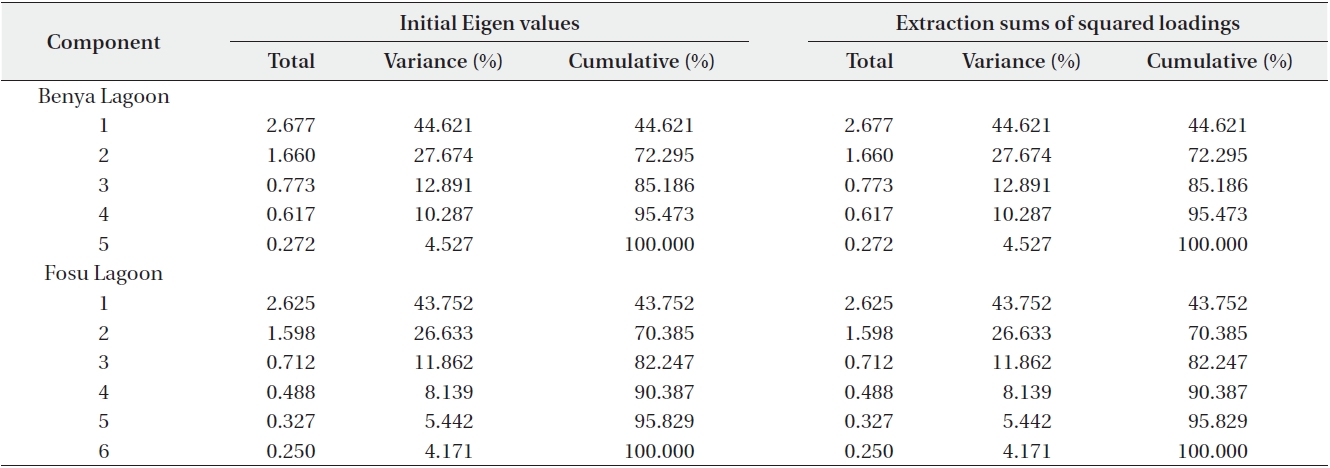
Total variance of Benya and Fosu lagoons data explained by principal component analysis (PCA)
due to the channeling of drainage systems into the lagoon (Armah et al. 2012). The level of DO in the closed Fosu Lagoon (less saline) was higher than that in the open Benya Lagoon (more saline). Our finding contradicts the results of Kwei (1977), which showed higher levels of DO in open lagoons, but was consistent with the findings of Davies et al. (2008). In all probability, reduced photoperiod and photosynthetic activities of aquatic plants account for the higher DO in the Fosu Lagoon. Salinity is also an important factor in determining the amount of oxygen that a body of water can hold. As the amount of dissolved salt in water increases, the amount of oxygen the water can hold decreases (Kennish 2002). The low salinity in the Fosu Lagoon compares well to that determined by Yankson and Kendall (2001) for the same lagoon, and is due to the fact that it does not have direct contact with the sea for several months in a year. The oxygen concentration ranged between 6.5 and 10.2 mg/L during the study. Decreased oxygen leads to excessive growth of aquatic weeds particularly
Effluents from agricultural and other human activities change the ambient conditions in the Fosu Lagoon since there is no seawater coming in within those times to dilute the system. The mechanic workshops near the Fosu Lagoon are sources of heavy metals and polycyclic aromatic hydrocarbons (Armah et al. 2012). The pH of the Fosu Lagoon is alkaline. pH affects metabolism and physiological processes of fish and also exerts considerable influence on the toxicity of ammonia and hydrogen sulphide, as well as the solubility of nutrients, and thereby water fertility (Lamptey and Armah 2008). The mean turbidity of both the Fosu and Benya lagoons were considerably lower than the results of Lamptey and Armah (2008) obtained for the Keta Lagoon. However, this was not true of conductivity, which was much higher in this study compared to the results of Lamptey and Armah (2008). Although the Keta Lagoon is considered to be an open lagoon, it is effectively closed for most of the year and is therefore similar to the Fosu Lagoon. Based on these anthropogenic pressures, the Fosu and the Keta lagoons are comparable.
The temperature range determined for the Fosu Lagoon was similar to results obtained by Lamptey and Armah (2008) for the Keta Lagoon. Aquatic plant species are dependent on growth conditions, particularly temperature, organic loading, oxygen status and nutrient availability. In the tropics, however, Edokpayi and Nkwoji (2007) argue that temperature is not an important ecological factor. The particle size composition plays an important role in the distribution of benthic macro fauna populations (Ruellet and Dauvin 2007). The Benya Lagoon substratum was composed of medium sand to coarse gravel, whereas the Fosu Lagoon was composed of coarse sand to very coarse gravel. Certain species (e.g., clams) occur in muddy sediments; others prefer coarse sediments such as amphipods and isopods. Fine sediments accumulate more pollutants and organic matter (Carvalho et al. 2006) and, therefore, are more susceptible to anoxic conditions, which limit the development of sensitive species (Dauer et al. 2000). In these conditions, macro fauna variability was likely not due to the granulometric factor, but to other factors, such as the point sources of pollutants, the degree of exposition, the water circulation, habitat type, and depth (Blanchet et al. 2008). Overall, the group with the highest percentage abundance was ecological group III. Total abundance was positively related to density of the dominant species. The survey of particular sampling stations proved that most of the stations had organisms or species that fell within ecological group III. Species from groups IV and V were not found at the Fosu Lagoon. These were second order opportunists, mainly small-sized polychaetes and first order opportunistic species, essentially deposit-feeders respectively. In the shallow coastal areas, the re-suspension and destabilization of sediments represent a more important disturbance to the benthic fauna (Burger and Gochfeld 2001).
The results of this study have several policy implications for the use and management of the two lagoons. Lagoon pollution is a multi-faceted environmental problem of considerable complexity. The success of lagoon conservation hinges on the prior existence of an adequate institutional/ governance environment for development of an integrated management scheme; and the cooperation of local governance structures. In any political strategy for lagoon management it is of paramount importance to make information available to: (i) support the assessments and assign priorities to environmental and socioeconomic concerns and issues; (ii) support research activities and development of the technical/scientific knowledge of the area studied (this is research-oriented); (iii) educational and awareness campaigns (e.g., classify and disclose lists of industries ranked by their environmental profiles); (iv) facilitate monitoring activities and enforcement (this is related to regulatory mechanisms); and (v) support decision making processes. These issues are relevant to the sustainable management of both lagoons. There is incentive to protect the lagoons because of the ecosystem services they provide (including the production of food and water; the control of climate and disease; nutrient cycles and crop pollination; and spiritual and recreational benefits). The direct beneficiaries will be the entire population, who will recover the right to have unpolluted water, a pleasant environment, and reduced health risks due to pollution.
The AMBI and multivariate statistics were used to determine the ecological health status of the Benya and Fosu lagoons in Ghana. The Benya Lagoon supported higher species richness and densities compared to the Fosu Lagoon. The Fosu Lagoon supported more pollution-tolerant organisms, such as the