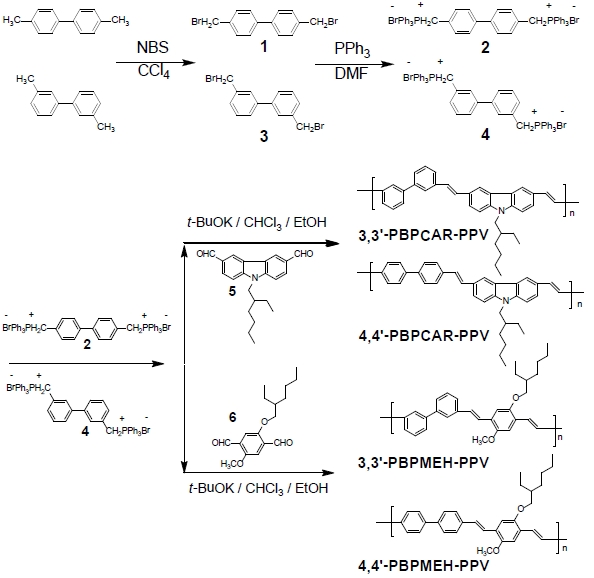
Electroluminescent devices generating light under applying the electrical bias are very promising for flat-panel displays. Demonstration of electroluminescence (EL) from organic molecules has accelerated the researches on light-emitting devices that utilize organic small molecules and conjugated polymers [1-4]. Since the first report of polymer light-emitting diodes based on poly(p-phenylenevinylene) (PPV) by the Cambridge group [1], a number of different polymers have been synthesized. Multicolor display applications require at least three basic colors: red, green, and blue. Among them, blue light, which is difficult to achieve with inorganic semiconductors such as GaN and ZnSe, has been sought in conjugated polymers. The feasibility and processibility of large-scale application using organic polymers spurred research into the use of conjugated polymers in blue light emission. Blue EL from partially eliminated PPV, poly(p-phenylene) (PPP), poly(alkylfluorene), and many types of copolymers were observed , but they still showed limited processibility and mechanical properties for the device [5-12]. The search for a stable, highly efficient, and pure blue light-emitting polymer still continues.
In this paper, we report on the syntheses and light-emitting properties of 4,4’- or 3,3’- linked biphenyl containing poly(pphenylenevinylene) related copolymers. We attempted to gain control of conjugation lengths and blue light emission by incorporating the kinked biphenyl unit into the polymer main chain. Using the 3,3’-biphenyl unit and a carbazole dialdehyde monomer, we successfully synthesized a blue light-emitting polymer through Wittig condensation polymerization. All the synthetic routes and polymer structures are shown in Scheme 1.
Materials. 4,4’-Dimethylbiphenyl, 3,3’-dimethylbiphenyl, carbazole, 4-methoxyphenol, 2-ethylhexylbromide, N-bromosuccinimide (NBS), triphenylphosphine (PPh3), and potassium
Instrumentation. The 1H NMR spectrum was recorded with a Bruker AMX 300 MHz spectrometer. UV-visible spectra of the 4,4’(3,3’)-PBPMEH-PPV and 4,4’(3,3’)-PBPCAR-PPV were measured on a Shimadzu UV-3100S. FT-IR spectra were measured by an EQUINOX 55 spectrometer. The thermogravimteric analysis (TGA) and differential scanning calorimetry (DSC) measurements of polymers were performed under nitrogen atmosphere, at a heating rate of 10 ℃/min with Dupont 9900 analyzers. Photoluminescence spectra of the polymers were obtained using a Spex Fluorolog-3 (model FL3-11) spectrofluorometer. EL spectra of the LED device were measured with a Minolta CS-1000 spectroradiometer. Luminance-currentvoltage (L-I-V) characteristics were recorded using a programmable current/voltage source (Keithley 238), and a luminance meter (Minolta LS-100).
Diphosphonium Salts Monomers. 4,4’-Bis(bromomethyl) biphenyl (1). To synthesize the compound 1, 1.47 g (8.0 mmol) of N-bromosuccinimide (NBS) was dissolved in 50 mL of CCl4 under nitrogen atmosphere. Then 0.72 g (4.0 mmol) of 4,4’-dimethylbiphenyl and a catalytic amount of benzoyl peroxide (BPO) were added to this solution and the mixture was heated 90℃ for 3 hr under nitrogen atmosphere. The completion of the reaction was identified by the appearance of succinimide on the surface of the reaction solution. The solution was extracted with methylene chloride and NaOH solution. The organic layer was washed with water, and dried over anhydrous MgSO4. The solvent was then removed by distillation to give 1.1 g (83.3%) of white solid. 1H NMR (CDCl3, ppm) δ 7.33-7.29 (m, 8H, aromatic), 4.54 (s, 4H, -CH2Br). 13C NMR (CDCl3,ppm): δ 141.0, 136.7, 130.0, 128.1, 33.3. Anal. Calcd. for C14H12Br2: C, 49.45; H, 47.00. Found: C, 49.90; H, 46.70.
4,4’-Biphenylbis(triphenylphosphonium bromide) (2). A solution of 1.0 g (2.9 mmol) of compound 1 and 1.83 g (7.0 mmol) of triphenylphosphine in 20 mL DMF was stirred and reacted at room temperature for 12 hr. The resulting mixture was poured into diethyl ether. After filtration and vacuum drying, diphosphonium salts monomer, 2, was obtained as a white solid. The product yield was 1.9 g (76%). 1H NMR (CDCl3 , ppm) δ 7.76-7.40 (m, 30 H, aromatic H), 7.34-7.27 (m, 8H, aromatic), 5.30 (d, 4H, -PCH2-).13C NMR (CDCl3,ppm): δ 137.06, 137.8, 136.0, 127.9, 129.3, 127.2, 58.4. Anal. Calcd. for C50H42Br2P2: C, 69.46; H, 4.90. Found: C, 69.10; H, 4.01.
3,3’-Bis(bromomethyl)biphenyl (3). To synthesize the compound 3, 1.47 g (8.0 mmol) of N-bromosuccinimide (NBS) was dissolved in 50 mL of CCl4 under nitrogen atmosphere. Then 0.72 g (4.0 mmol) of 3,3’-dimethylbiphenyl and a catalytic amount of benzoyl peroxide (BPO) was added to this solution and the following synthetic procedures were performed in the same manner as for compound 1. The product yield was 0.9 g (68.2%). 1H NMR (CDCl3, ppm) δ 7.56-7.36 (m, 8H, aromatic), 4.57 (s, 4H, -CH2Br). 13C NMR (CDCl3, ppm): δ 139.9, 138.7, 130.5, 129.4, 128.1, 127.8, 33.5. Anal. Calcd. for C14H12Br2: C, 49.45; H, 47.00. Found: C, 49.01; H, 46.90.
3,3’-Biphenylbis(triphenylphosphonium bromide) (4). A solution of 1.0 g (2.9 mmol) of compound 3 and 1.83 g (7.0 mmol) of triphenylphosphine in 20 mL DMF was stirred and reacted at room temperature for 12 hr. The following synthetic procedures were performed in the same manner as for compound 2. The product yield was 1.6 g (64%). 1H NMR (CDCl3 , ppm) δ 7.81- 7.45 (m, 30 H, aromatic H), 7.31-7.21 (m, 8H, aromatic), 5.26 (d, 4H, -PCH2-). 13C NMR (CDCl3, ppm): δ 141.3, 137.2, 136.0, 130.6, 128.4, 127.5, 128.8, 128.6, 58.7. Anal. Calcd. for C50H42Br2P2: C, 69.46; H, 4.90. Found: C, 69.05; H, 4.60.
Dialdehyde Monomers. 9-(2-Ethylhexyl)carbazole-3,6-dicarbaldehyde (5). This was synthesized by an adapted literature procedure, and as in our previous work [13].
5-(2-Ethylhexyloxy)-2-methoxybenzene-1,4-dicarbaldehyde (6). This was synthesized by an adapted literature procedure, and as in our previous work [14].
Polymerization of 4,4’(3,3’)-PBPCAR-PPV. A solution of 0.38 g (1.15 mmol) of 9-(2-ethylhexyl)carbazole-3,6-dicarbaldehyde and 1 g (1.15 mmol) each of diphosphonium salt monomer (4,4’ -biphenylbis(triphenylphosphonium bromide) for 4,4’-PBPCARPPV, and 3,3’- biphenylbis(triphenyl phosphonium bromide) for 3,3’-PBPCAR-PPV), was prepared in 10 mL of chloroform. 0.67 g (6 mmol) of potassium t-butoxide was dissolved completely in 10 mL of ethanol, and this base solution was carefully dropped into the solution of monomers. After 1-2 days, the polymer product was precipitated from methanol. The crude polymers were filtered, and then purified by using a Soxhlet extractor in methanol for 2 days. The polymer yields were 0.31 g (56.0%) for 4,4’-PBPCAR-PPV, and 0.35 g (63.1%) for 3,3’-PBPCAR-PPV.
Polymerization of 4,4’(3,3’)-PBPMEH-PPV
A solution of 0.34 g (1.15 mmol) of 5-(2-ethylhexyloxy)-2-methoxybenzene- 1,4-dicarbaldehyde and 1 g (1.15 mmol) each of diphosphonium salt monomer (4,4’-biphenylbis (triphenylphosphonium bromide) for 4,4’-PBPMEH-PPV, and 3,3’-biphenylbis (triphenylphosphonium bromide) for 3,3’-PBPMEH-PPV), was prepared in 10 mL of chloroform. 0.67 g (6 mmol) of potassium t-butoxide was dissolved completely into 10 mL of ethanol, and this base solution was carefully dropped into the solution of monomers. After 1-2 days, the polymer product was precipitated from methanol. The crude polymers were filtered, and then purified by using a Soxhlet extractor in methanol for 2 days. The polymer yields were 0.32 g (61.5%) for 4,4’-PBPMEH-PPV, and 0.33 g (63.5%) for 3,3’-PBPMEH-PPV.
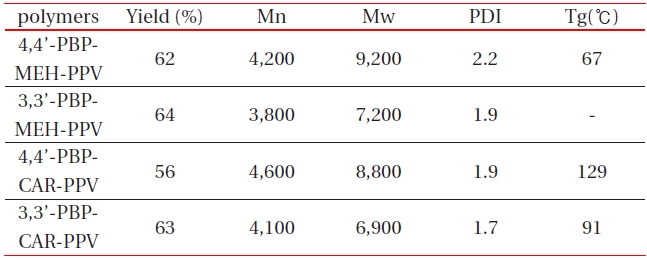
[Table 1.] Polymerization results of 4,4’(3.3’)-PBPMEH-PPV and 4,4’(3.3’)-PBPCAR-PPV .
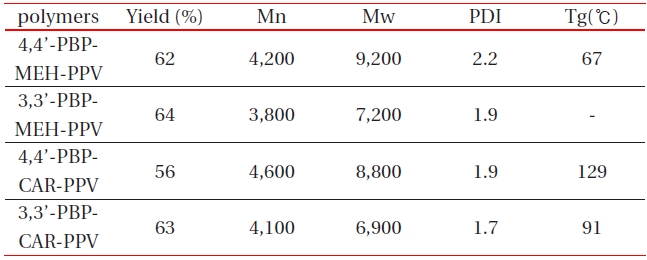
Polymerization results of 4,4’(3.3’)-PBPMEH-PPV and 4,4’(3.3’)-PBPCAR-PPV .
All of the synthesized polymers were highly soluble in common organic solvents such as tetrahydrofuran, chloroform, methylene chloride, and 1,2-dichloroethane. They could be spincast onto various substrates to give highly homogeneous thin films without heat treatment. The polymerization results of all synthesized polymers are summarized in Table 1. The numberaverage molecular weight (
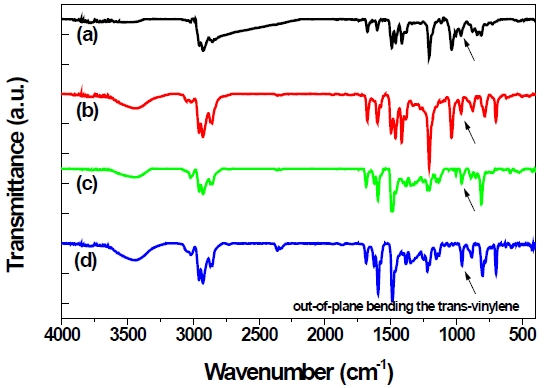
Figure 1 shows the typical FT-IR spectra of 4,4’ (3,3’)-PBPCARPPV and 4,4’ (3,3’)-PBPMEH-PPV. All polymers show weak but clear absorption peaks at about 960 cm-1 corresponding to the appearance of the out-of-plane bending mode of the
ylene. This proves that the vinylene double bond formation and consequently the polymerization reaction, has been successful [9,10].
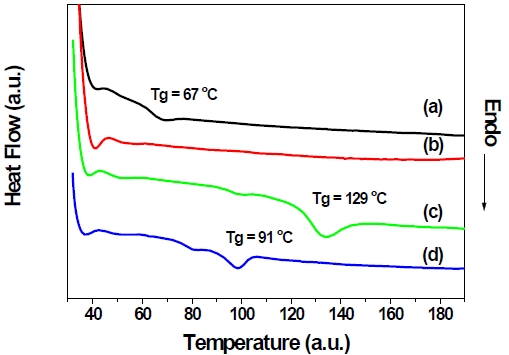
The thermal properties of the synthesized polymers were evaluated by means of TGA and DSC under nitrogen atmosphere. All synthesized polymers showed good thermal stability up to 300℃ in TGA thermograms (not shown here). Figure 2 shows DSC thermograms of polymers; 4,4’-PBPCAR-PPV shows the highest
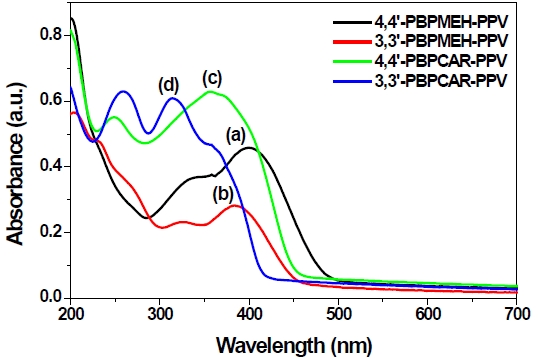
Figure 3 shows the UV absorption spectra of 4,4’(3,3’)-PBPCAR- PPV and 4,4’ (3,3’)-PBPMEH-PPV in a thin film onto a quartz plate. The absorption maximum of the well known polymer MEH-PPV is known to be at about 510 nm. However, as shown in the absorption spectra, 4,4’-PBPMEH-PPV showed a 400 nm absorption maximum, due to the biphenyl unit in the alternating copolymer system. For 3,3’-PBPMEH-PPV, a maximum absorption wavelength appeared at 385 nm. This means that the π-electron delocalization of the polymer main chain was more interrupted by 3,3’-linkage than by 4,4’-linkage, yielding a reduction of the π-conjugation length. The carbazole containing alternating copolymers, 4,4’(3,3’)-PBPCAR-PPV showed more blue shifted absorption maxima than did 4,4’ (3,3’)-PBPMEH-PPV.
The maximum absorption wavelengths of 4,4’-PBPCAR-PPV
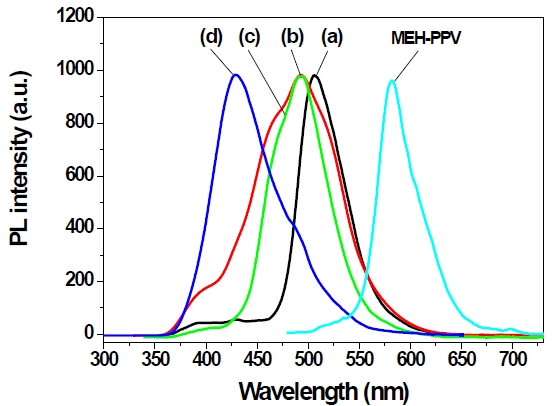
and 3,3’-PBPCAR-PPV are 361 and 314 nm, respectively. This means that carbazole linkages could more effectively reduce the conjugation length than could phenyl linkages of PBP-MEH-PPV polymers. The PL spectra showed drastic changes of emission color, because of 3,3’ or 4,4’-biphenyl and the carbazole linkage. The PL spectra of the well known MEH-PPV, 4,4’(3,3’)-PBPMEHPPV and 4,4’(3,3’)-PBPCAR-PPV polymer thin films coated on quartz plates are shown in Figure 4.
The PL spectra of the well known MEH-PPV [10] showed that the emission maximum is about 580 nm. The emission of 4,4’-PBPMEH-PPV was blueshifted by about 73 nm, because of the unsubstituted biphenyl unit in the alternating copolymer system. For the case of 3,3’-PBPMEH-PPV, the emission peak was more blueshifted compared to that of 4,4’-PBPMEH-PPV, and showed at around 493 nm, due to the more bended polymer structure caused by 3,3’-biphenyl linkage. The carbazole containing 4,4-PBPCAR-PPV exhibited a similar emission maximum at about 493 nm as the emission maximum of 3,3-PBPMEHPPV. We found that the carbazole group and 3,3’-biphenyl linkages effectively reduced the conjugation length of the polymers; therefore, 3,3’-PBPCAR-PPV showed a PL emission maximum at 430 nm. Consequently, we synthesized pure blue light-emitting conjugated polymers, by introducing the biphenyl linkages and carbazole group into the polymer main chain.
A single-layer light-emitting diode was fabricated by using ITO as the anode and Al as the cathode, respectively. 4,4’(3,3’)-PBPMEH- PPV and 4,4’(3,3’)-PBPCAR-PPV polymers were deposited onto indium-tin oxide-covered glass substrates, by spin-casting the soluble polymers in 1,2-dichloroethane. The spin-casting technique yielded uniform films with thicknesses of about 60-70 nm. The Al electrode (cathode) was then evaporated onto this
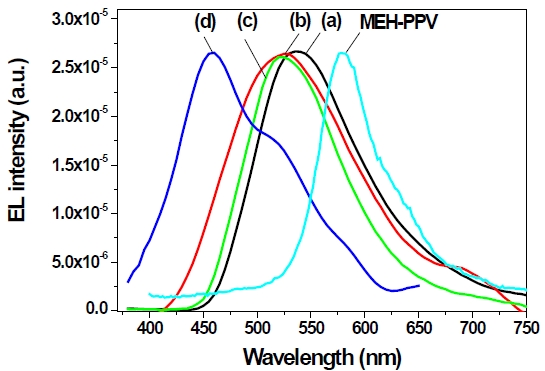
This result means that the π-conjugation length could be systematically controlled by 3,3’-biphenyl linkage and the carbazole group, and therefore blue EL emission was successfully obtained. These results indicate that biphenyl and carbazole containing PPV derivatives could be good candidates for blue-emitting ma-
terial.
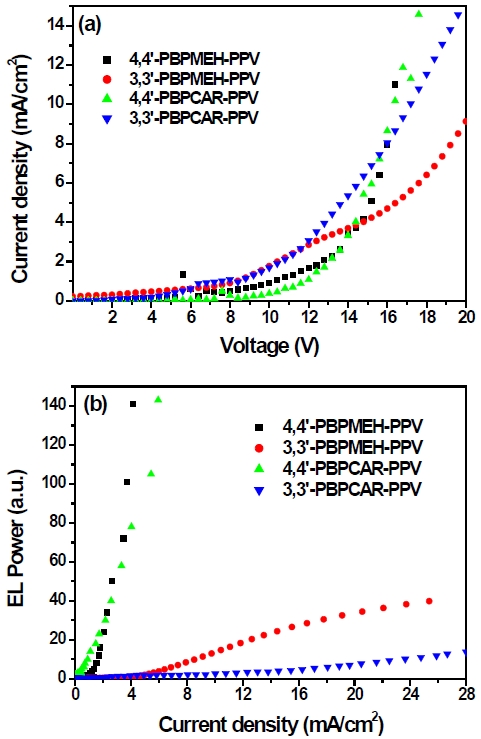
Figure 6 (a) shows the current density vs. voltage characteristics measured from all the synthesized polymers. The forward current increases with increasing forward bias voltage for all devices. The threshold voltages of all polymers were in the range 7~8 V. Figure 6 (b) exhibits the emission intensity vs. current density characteristics from 4,4’-PBPMEH-PPV, 3,3’-PBPMEH-PPV, 4,4’-PBPCAR-PPV, and 3,3’-PBPCAR-PPV devices, respectively. The EL efficiencies of polymers with a 4,4’-linked naphthalene unit are relatively higher than those of polymers with a 3,3’-linked naphthalene unit, at the same current density. Further device optimizations and electrical characterizations of the synthesized polymers are in progress, by replacing the cathode electrode as LiF/Al or Ca/Al, or the insertion of a PEDOT layer as a hole injection layer, to increase the EL efficiency of synthesized polymers.
We synthesized alkoxy-substituted phenyl or carbazole containing 4,4’(3,3’)-PBPMEH-PPV and 4,4’(3,3’)-PBPCAR-PPV polymers through the well-known Wittig condensation polymerization. The π-conjugation lengths of synthesized polymers were regulated by 4,4’- or 3,3’ linked biphenyl groups. All polymers were completely soluble in organic solvents, and could be made as homogeneous thin film, without heat treatment. In the UVvisible and PL spectra, the maximum absorption and PL emission spectra were systematically blueshifted, because of the 4,4’- or 3,3’-linked biphenyls and carbazole group. Consequently 3,3’-PBPCAR-PPV showed blue emission at 430 nm. A singlelayer light-emitting diode of ITO/3,3’-PBPCAR-PPV/Al was fabricated. The EL spectrum of 3,3’-PBPCAR-PPV gave the highest peak at 455 nm, which corresponds to pure blue emission. Carbazole containing PPV derivatives linked by biphenyl groups could be a promising candidate for blue-emitting polymers.