



Hydroperoxyl radical/superoxide anion radical (HO2·/O2 -·, p
In order to elucidate characteristics of HO2·/O2 -·, various methods have been used in the detection of HO2·/O2 -·. The most direct techniques of these can be electron spin resonance (ESR) and the optical absorption detection by means of spin trapping and characteristic UV spectra of HO2 and O2 - (240?260 nm), respectively [3,17]. However, ESR can be detected only in ice at very low temperature, whereas UV absorbance can lead to the possibility of complicated reactions arising from interference of the solute, i.e., spectrum overlapping as many chemical species, with the primary processes [3,18]. Hence, these methods are of very low sensitivity and low selectivity in practical applications [5]. The most widely used method of detecting HO2·/O2 -· is through the use of such tetranitromethane, nitroblue tetrazolium, and cytochrome C which form products with intense optical absorbance [3]. However, these spectrophotometric methods have also suffered from low sensitivity [5,19,20].
Recent studies have reported a chemiluminescent method using luminol and enzyme, which are disadvantageous as these reagents are unstable and expensive [21-23]. In addition, Kwon and Lee [5] have reported a kinetic method by HO2·/O2 -·-driven Fenton-like chemistry in UV/H2O2 process, which detected only o- and m-hydroxybenzoic acid (OHBA isomers) as fluorescent hydroxylated derivatives produced from benzoic acid (BA). However, the kinetic method for HO2·/O2 -· by using BA as a probe has produced various products including p-OHBA, decarboxylated product (i.e., phenol), and dihydroxybenzoic acid (i.e., 2,3- , 2,4-, and 2,5-diOHBA), and so on, which would interfere with the analysis of o- and m-OHBA isomers [24-26]. First above all, these products could not be detected in the fluorometer employed in the kinetic method. As a result, the sensitivity of the kinetic method by using BA probe might be decreased by various products. In continuation of our earlier works on the kinetic method by using BA [5], we studied the enhanced sensitivity for determining HO2·/O2 -· compared to BA. Therefore, an alternative method of detecting HO2·/O2 -· with high sensitivity is necessary to be developed to elucidate characteristics of HO2·/O2 -· having
very low concentration in oxidation processes.
In the present paper, we reported investigations on a more advanced kinetic method for HO2·/O2 -· determination by employing terephthalate or terephthalic acid (TA) as a probe. TA was used to trap ·OH in that TA might produce only hydroxyterephthalic acid or hydroxyterephthalate (OHTA) in the calibration procedure, which can be determined by fluorescence measurements.
BA, 3%H2O2, NaOH, Fe(III)-ethylenediaminetetraacetic acid (Fe3+ -EDTA), HCl, and so on were the American Chemistry Society (ACS) grade (Sigma-Aldrich, St. Louis, MO, USA) and used as received. Owing to its impurity, the purification of disodium terephthalate (Aldrich, 96%) was checked by UV/visible spectrophotometer (UV1601; Shimazu, Kyoto, Japan) that had been recrystallized several times with hot water and then by drying in a dry oven (60 ± 5℃), and no impurities were observed.
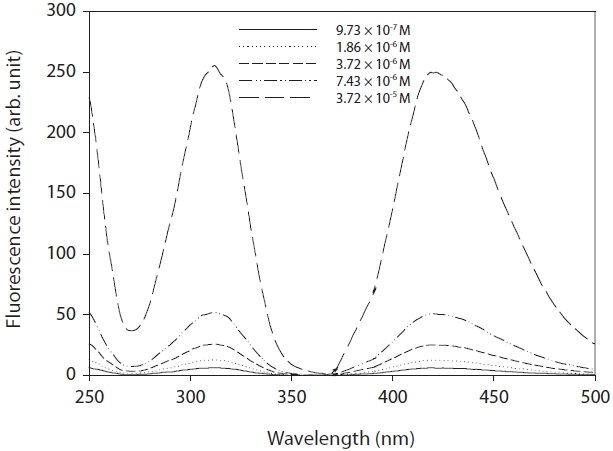
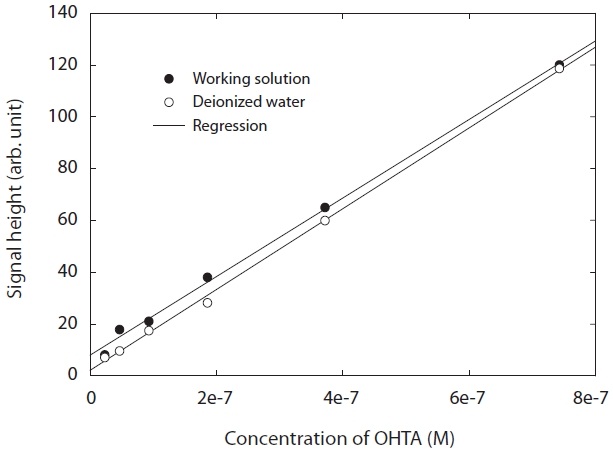
A commercial product of OHTA failed to find information on its properties. Hence, OHTA was synthesized from previous studies [27-29]. The OHTA synthesized could be checked by the spectra of a fluorescence detector (LS 50B; Perkin-Elmer, Buckinghamshire, UK), as shown in Fig. 1. Furthermore, as shown in Fig. 2, the OHTA synthesized showed the linearity of the response of the measurement systems with fluorescence detector with a good correlation coefficient (R2 = 0.997), which was performed with working solutions and pure deionized water instead of working solutions, respectively. In this study,synthesized was used to only identify OHTA formation with a fluorescence detector (474 model; Waters, Milford, MA, USA) in the apparatus used in this study.
All experiments were conducted with proper buffer solutions, ammonium acetate buffer, acetate buffer, borate buffer and carbonate buffer, by addition of the respective 1 M H2SO4 or 0.5 M NaOH because experimental solution on the pH-dependent rate constant (kobsd) [3] should be kept at constant pH during the reaction of HO2·/O2 -· in actual analysis. All solutions were prepared in
triple deionized water, which was further purified by an aqua- Max water system (Young Lin Co., Anyang, Korea).
2.2. Experimental Procedures and Analyses
The apparatus and procedures for HO2·/O2 -· measurement employed in the present study was almost similar to a previous study [5], except for a home-made glass debubbler. Briefly, all solutions were delivered by using a peristaltic pump (Ismatec, Glattbrugg, Switzerland) with polytetrafluoroethylene (PTFE) tubing (Cole-Parmer, Vernon Hills, IL, USA; i.d., 0.8 mm). The H2O2 solution (0.42 mL/min) was photolyzed by UV irradiation, which was equipped with a 4 W low pressure Hg lamp (λmax = 254 nm; Philips, Amsterdam, Netherlands). The HO2·/O2 -· stream produced from the photolysis of H2O2 in a quartz coil-type reactor was passed through a knotted tube reactor (KTR), which was able to control the concentration of HO2·/O2 -· and, at the same time, to completely premix the solutions.
Then, Fe3+-EDTA solution (0.23 mL/min) was mixed with TA solution (0.23 mL/min) in KTR and then joined with the HO2·/O2 - ·-H2O2 stream. The reduction of Fe3+-EDTA by HO2·/O2 -· resulted in the production of Fe2+-EDTA, which reacted further with residual H2O2. A Fenton-like reaction of H2O2 and Fe2+-EDTA produced the ·OH radicals, which were then scavenged by TA to produce hydroxylated products in KTR. After 0.05 N NaOH (0.23 mL/min) was added to raise the pH level above 11, the fluorescence intensity of OHTA could be maintained at a maximum level.
The mixed solution occasionally caused the formation of air bubbles in the effluent stream. Air bubbles were then removed by a home-made glass debubbler prior to the entry of a fluorometer in order to prevent noise signal by the air bubbles. The fluorescent signal was transferred to a data acquisition system, Autochro-Win (Young Lin Co.), consisting of an analog-to-digital converter with a personal computer.
The OHTA fluorescence was monitored with a fluorometer (Waters 474 model) equipped with a 16 μL flow-through cell using 315 nm (excitation)/425 nm (emission) with slit-width of 40 nm. The fluorescent intensity of the OHTA was recorded with the fluorescence detector as a function of time.
The OHBA fluorescence was measured with a fluorometer equipped with a 16 μL flow-through cell using 320 nm (excitation)/400 nm (emission) with a slit-width of 40 nm.
The prominent feature of the kinetic method was the ability to calibrate the concentration of HO2 ·/O2 -· without a primary standard [5]. Briefly, to calibrate the HO2·/O2 -· measurement system, all working solutions were passed through the appropriate ports under UV lamp-off and the base lines were monitored. The H2O2 solution placed in UV photolysis under UV lamp-on was photolyzed and then passed through the gradient-length of KTR, which was controlled step-wise as 0, 1, 2, 3, and 4 m. In the absence of additives, HO2· and O2 -· in each KTR were disproportionated by self-reactions of R3-R5 (Table 1) according to the empirically observed pH-dependent rate constant, kobs [3], assuming that reaction R5 is negligible due to its very small rate constant (k5 < 0.3/M/sec):
where kobs can be calculated using k3 = (8.3 ± 0.7) × 105/M/sec, k4 = (9.76 ± 0.6) × 107/M/ s, and KHO2 = 1.6 × 10-5/M as recommended values [3]. A stream containing HO2· and O2 -· produces ·OH by the mechanism including a Fenton-like reaction. In this manner, each fluorescence signal of OHTA produced from the ·OH radical reaction with respective TA was proportional to a produced HO2·/O2 -· concentration. The half-life (t1/2) of the HO2·/O2 -· was obtained by plotting a simple linear relationship of the signal ratio vs. reaction time. The half-life in a second-order reaction was inversely proportional to the initial concentration. Thus, the initial concentration of HO2·/O2 -· could be kinetically calculated from the disproportionation reaction of HO2·/O2 -· based on the half-life and calculated kobs at a given pH.
The pH was measured by pH meter (Model PCM700; Orion Research Inc., Jacksonville, FL, USA).
Table 1 shows the reaction scheme considered from this study. As shown in Table 1, the reaction scheme was comprised of the previously reported reactions and second-order rate constants (k). In the R1 ofion scheme, H2O2 was photo-decomposed to produce two ·OH radicals by an UV irradiation at wavelength 254 nm in the quartz coil-type reactor. Most of the ·OH radicals formed in the quartz coil-type reactor under UV irradiation could react with residual H2O2, producing HO2·/O2 -·
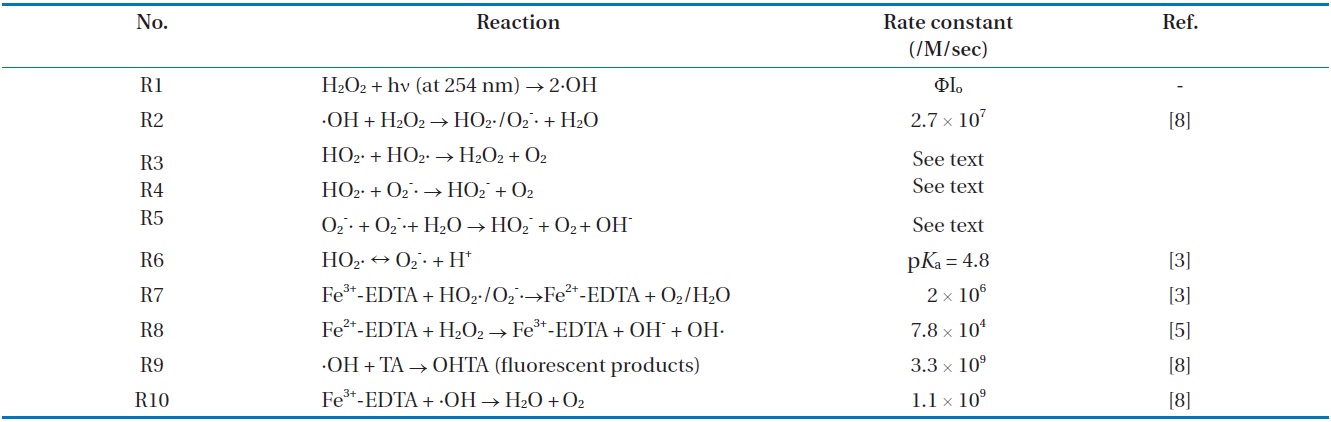
Reaction scheme
(R2). Hence, the HO2·/O2 -· stream was produced from the photolysis of H2O2 only in a quartz coil-type reactor.
Hydroperoxyl radical formed from the quartz coil-type reactor is dependent upon the acid-base equilibrium with p
where H is Henry’s law coefficient (M/atm) of HO2· at 25℃, and K is the equilibrium constant from R4 in Table 1. Since uncertainty in H (1.2 ~ 6.8 × 103 M/atm) might be somewhat [31,32], we recalculated H* with updated datum of H (2.0 × 103 M/atm) [33]. As shown in Fig. 3, for pH values smaller than 5, HO2·/O2 -· does not dissociate appreciably and its effective Henry’s law constant is
equal to its Henry’s law constant. However, for pH values larger than 5, the effective Henry’s law constant of HO2·/O2 -· depending on various pH values increased with pH, and thus its solubility and mobility would increase with pH [7].
Fe3+-EDTA could alter the reactions of R3 to R5. Fe3+ -EDTA was reduced by HO2·/O2 -· to produce Fe2+ -EDTA and O2 with R7 [5]. Fe2+-EDTA and H2O2 led to the production of the ·OH radical and regeneration of Fe3+-EDTA in R8. Then, the ·OH radical produced OHTA in the presence of TA with a nearly diffusion-controlled rate constant of k9 = 3.3 × 109/M/sec (R9) [8]. Fe3+-EDTA, however, may compete with 1 mM TA for the ·OH radicals. To minimize the scavenging of ·OH radicals by Fe3+-EDTA, a low concentration of 20 μM Fe3+ -EDTA to keep the ratio of k9[TA] to k10[Fe3+-EDTA] larger than 150 was maintained. In other words, about 99.34% of the ·OH radicals will kinetically react with TA, and other competitive reactions for ·OH radicals would be negligible.
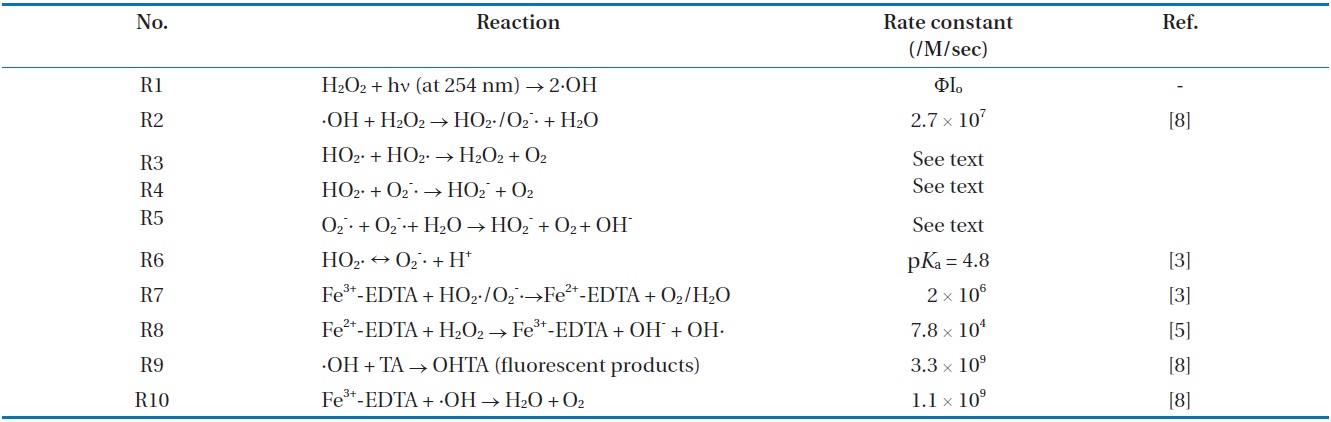
In the meantime, in the first paper by Kwon and Lee [5], the kinetic method adopted BA as a probe was used to demonstrate the HO2·/O2 -· detection system. Although the new setup as described in Kwon and Lee [5] has the advantage that the kinetic method was very sensitive, it introduced the potential loss of p- OHBA as a product on the detection of HO2·/O2 -· as well as the interference of by-products, which would lead to an error in the determination of HO2·/O2 -· by using BA in the kinetic method. As shown in Fig. 4, the HO2·/O2 -· loss in the detection system by using BA existed, compared to using TA. Hence, HO2·/O2 -· concentrations measured by using BA were lower than those using TA. In this case, the sensitivities of the kinetic method by using TA were always higher with 1.7?2.5 times at pH 8.0 than those by using BA (Fig. 4). This result suggests that ·OH trapping with TA instead of BA can be effective. Therefore, the magnitude of this loss on HO2·/O2 -· can be substituted with OHTA using TA as a probe of ·OH.
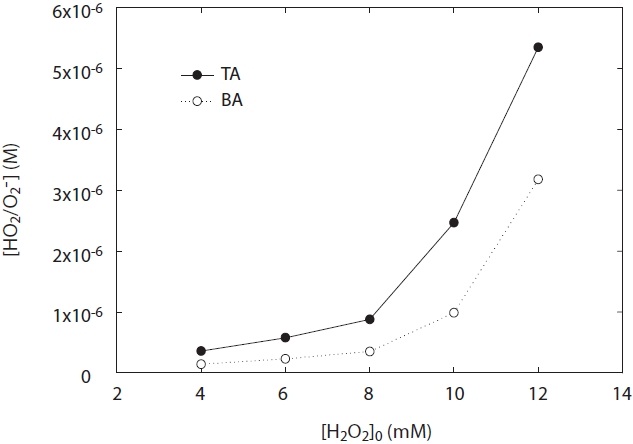
Fig. 5 shows the concentration of HO2·/O2 -· generated from H2O2 photolysis at a wavelength 254 nm under two experimental conditions: pH and H2O2 concentration. As shown in Fig. 5, the concentration of HO2·/O2 -· at the ranges of pH 5?10 was dependent upon both various pH values and H2O2 concentrations added. The concentration of HO2·/O2 -· increased with pH value, and its concentration measured at pH 5 was lower than that at pH 10. This result suggests that O2 -· slowly decayed at high pH
[Fig. 5.] HO2·/O2 -· concentration depending on H2O2 concentration and pH. terephthalic acid = 1 mM.
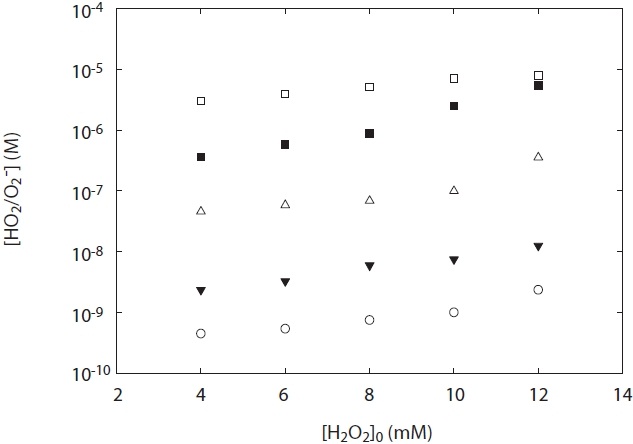
conditions. To the best of our knowledge, one of the plausible explanations for the decay of HO2·/O2 -· is the kobs on the disproportionation reactions of R3?R5 and the pH dependences of the acid-base equilibrium between HO2· and O2 -·. As shown in Table 1, self-disproportionation by reactions R3?R4 will be fast and dominant at an acidic pH range, whereas disproportionation by reaction R5 at high pH ranges (pH > 7) is very slow with its kobs. Hence, there is a low loss of HO2·/O2 -· concentration at high pH ranges and then its concentration is relatively high. These results are consistent with the kinetic data for the disproportionation reaction of HO2·/O2 -· presented by Bielski et al. [3].
For a more profound account for the pH dependence of HO2·/ O2 -· concentrations generated in a continuous flow system, a series of experiments were carried out over ranges of pH 2?10 and a range of initial H2O2 concentration (4 mM). In each experiment, the life time of HO2·/O2 -· formed during H2O2 photolysis was investigated on the basis of the kinetic method over various pH conditions.
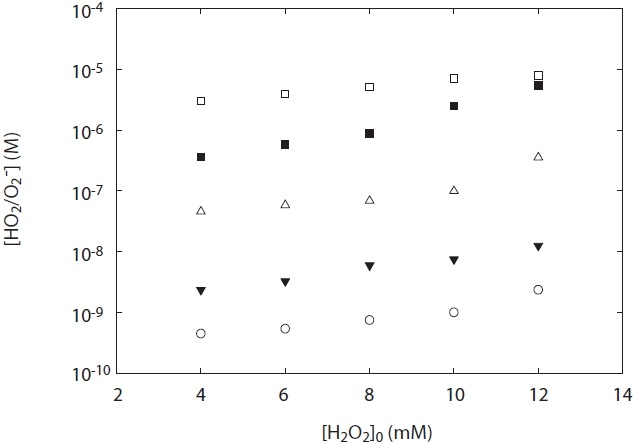
Fig. 6 shows the life-time of HO2·/O2 -· as a function of solution pH at 4 mM H2O2 concentration. As shown in Fig. 6, its life times were the lowest with approximately 51 sec at pH 4.8?5.0 (= near p
From Eq. (3), t1/2 can readily be determined from Eq. (4) at a given pH [5]. Finally, the life time (t) of HO2·/O2 -· is Eq. (5) at a given pH.
As a result, the life time of HO2·/O2 -· as well as it concentration was highly pH dependent. In particular, at ranges of pH
2?10, its life-time was approximately from 51 to 422 sec as shown in Fig. 6. In particular, the increase in the life-time with pH was observed as expected. This result is consistent with the fact that is estimated with the observed second-order rate constants for spontaneous decay of HO2·/O2 -· by Bielski et al. [3]. At high pH > 8, the life-time of O2 -· can be estimated to be larger than 100 sec, if [HO2·/O2 -·] > 10-6 M. McDowell et al. [17] presented from 90 sec at pH 11.0 to 41 min at pH 12.5 with the half-time for decay of 100 μM O2 -·. Bieski et al. [3] observed that, at pH 10, relatively slow rate processes for HO2·/O2 -· reaction could be determined because the first half-life for spontaneous disproportion of O2 -· was relatively long into 70?100 sec. Although a little difference of the life-time of HO2·/O2 -· between measured and predicted exists, this result can be acceptable. The chemical life-time of O2 -· at pH > 6 increases proportionally with pH, whereas its lifetime at pH < 4 increases inversely with decreasing pH. However, at about pH 4?6, the chemical life-time of HO2·/O2 -· is relatively short because of the spontaneous disproportionation reaction. Thus, these results suggest that the chemical life-time of HO2·/O2 -· is dependent upon the kinetics with the observed secondorder rate constant of HO2·/O2 -·.
We have newly adopted TA as a probe for the measurement of HO2·/O2 -· in the kinetic method presented in our previous study. Our study results showed that concentrations of HO2·/O2 -· formed from the photolysis technique of H2O2 at a range of pH 2?10 increased with pH. In addition, an increase in the life-time of HO2·/O2 -· with pH was observed. Since the kinetic method by using TA was highly sensitive, this study can contribute to understanding the basic function of HO2·/O2 -· in advanced oxidation processes.
![pH dependences of the empirically observed pH-dependent rate constant (kobs), effective Henry’s law coefficient, and molar ratio of [HO2·]/[O2 -·] or [O2 -·]/[HO2·] on various pH values.](http://oak.go.kr/repository/journal/11761/E1HGBK_2012_v17n4_205_f003.jpg)
![pH dependence on the life-time of HO2·/O2 -·. [H2O2]o = 4 mM.](http://oak.go.kr/repository/journal/11761/E1HGBK_2012_v17n4_205_f006.jpg)