



Many researchers have studied that many processes to effectively remove heavy metals in water/wastewater. Especially, among many processes, physical and chemical processes are relatively simple and obtain high treatment efficiency for removal heavy metals compared with biological treatment. Recently, interests in physical and chemical methods are sharply increasing again because of dangerousness for radioactive element. In this study, various physical and chemical processes such as chemical precipitation, ion-exchange, electrodialysis, and membrane separation are introduced.
연구자들은 용수 및 폐수 내에 함유되어 있는 중금속을 효과적으로 제거하기 위한 많은 공정을 연구하고 있다. 많은 공정들 중에서 특히, 물리, 화학적 공정은 생물학적 공정과 비교해볼 때 대체로 간단하고 높은 중금속 제거효율을 얻을 수 있다. 최근에는 방사능원소에 대한 위험성 때문에 물리, 화학적 방법들에 대한 관심이 다시 급격하게 증가하고 있다. 이 연구에서는 화학적 침전, 이온 교환, 전기투석, 그리고 막 분리 등과 같은 다양한 물리, 화학적 공정들을 소개하고자 한다.
The concentrations of heavy metals must be reduced to very low levels prior to release of the wastewater, because of the toxicity of many heavy metals. Especially, the removal for radioactive metals such as cesium and strontium is of increasing concern among many researchers[1]. Although many processes for heavy metals treatment have studied and applied more efficient removal processes for heavy metals are needed for recycling of water, strict regulation for the effluent concentration of heavy metals, and reducing operation cost of the process for recycling of precious metals. Each physical and chemical treatment process has their own advantages and disadvantages and understanding these factors is useful for selection and application to the specific case. In this study, various physical and chemical processes will be introduced.
2. Physical and Chemical Treatment Process
The most widely used process for removal of heavy metals from solution is chemical precipitation. Patterson and Minear[2] reported that approximately 75.0% of the electroplating facilities employed precipitation treatment, using either hydroxide, carbonate, or sulfide treatment, or some combination of these treatments to treat their wastewater. The most commonly used precipitation technique is hydroxide treatment due to its relative simplicity, low cost of precipitant (lime), and ease of automatic pH control. The solubilities of the various metal hydroxides are minimized for pH in the range of 8.0 to 11.0. Iron manganese, copper, zinc, nickel, and cobalt result in almost complete removal by hydroxide precipitation with almost no special modification required. However, precipitation of mercury, cadmium, and lead may be slow and incomplete. When chromium is present, reduction of the solution with sodium metabisulfide, ferrous sulfate, or metallic iron prior to lime treatment is necessary. To reduce hexavalent chromium to the trivalent form, chrome bearing streams are generally segregated and treated separately. Chlorination is sometimes required to break down the complexed organic metallic compounds prior to chemical precipitation. Employing hydroxide precipitation at elevated pH provides conditions where the metal hydroxides have low solubilities and precipitate out on settling, typically over time periods of 2 to 4 hours. Hydroxide precipitation of heavy metals is well suited for automatic pH control and has been shown to be an effective treatment technique in industry. However, limitations associated with the use of hydroxide treatment include as follows:
(1) Hydroxide precipitates tend to resolubilize if the pH solution is changed
(2) Cr6+ ion is not removed by hydroxide precipitation
(3) Removal of metal hydroxide precipitation of mixed metal wastes may not be effective because the minimum solubilities for different metals occur at different pH condition
(4) The presence of complexing agents may have an adverse effect on metal removal
(5) Cyanide interferes with heavy metal removal by hydroxide precipitation
(6) Hydroxide sludge quantities can be substantial and are generally difficult to dewater due to the amorphous particle structure
Carbonate precipitation has several advantages over those of conventional hydroxide precipitation.
(1) Optimum carbonate precipitation treatment occurs at lower pH conditions than those for optimum hydroxide treatment.
(2) Metal carbonate precipitates are reported to be denser than the hydroxide precipitate causing improved solids separation.
(3) Carbonate sludge have better filtration characteristics than hydroxide sludge.
No advantage in terms of denser sludge or better filtration characteristics were observed for the zinc carbonate and nickel carbonate systems over the corresponding hydroxide systems. Sulfide precipitation has been demonstrated to be an effective alternative to hydroxide precipitation for removal of heavy metals from industrial wastewaters[3]. Attractive features of this process include:
(1) Attainment of a high degree of metal removal even at low pH (pH range: 2.0 to 3.0)
(2) Low detention time requirements in the reactor because of the high reactivity of sulfides
(3) Feasibility of selective metal removal and recovery
(4) Metal sulfide sludge is three times less subject to leaching at pH 5.0 as compared to metal hydroxide sludge making final disposal safer and easier
(5) Metal sulfide sludge exhibit better thickening and dewatering characteristics than the corresponding metal hydroxide sludge
Limitations of the process include the potential of H2S gas evolution and the concern for sulfide toxicity. Eliminating of sulfide reagent prevents formation of the odor causing H2S. In currently operated soluble sulfide systems which do not match demand must be enclosed and vacuum evacuated to minimize sulfide odor problems. Generally, precipitation has been widely used for their simplicity but need to be replaced by other processes due to following disadvantages[4].
(1) It usually result in a net increase in the total dissolved solids of the wastewater being treated
(2) Large amount of sludge requiring treatment, which, in turn, may contain toxic compounds that may be difficult to treat
Recently, Fenglian et al.[5] applied advanced Fenton- chemical precipitation to the treatment of strong stability chelated heavy metal containing wastewater.
In general, ion exchange is well known as technique for softening and purification of water. Also, it is an effective means of removing heavy metals from wastewater. It has been used to recover valuable metal such as silver or chrome ions and treat wastewater, which is polished. It is a reversible chemical reaction, where the removal of heavy metals is accomplished by the exchange of ions on the resin for those in wastewater. When the resins are saturated, they must be regenerated with an acid or an alkaline medium to remove the metal ions from the resin bed. The regenerant brine is smaller in volume and higher in concentration than the original wastewater, but these metals must be adequately treated or recovered. Due to the fact that ion exchange is efficient in removal of dissolved solids from normally dilute spent rinse waters, it is well suited for use in water purification and recycling. Many of the plating chemicals, acids, and bases used in metal finishing are ionized in solution and can be removed by ion exchange. Factors making ion exchange effective for such an application include:
(1) Ion exchange can economically separate dilute concentrations of ionic species from solutions
(2) The process can consistently provide high purity water over a broad range of conditions
(3) The resins used for separation are durable under severe chemical environments
In comparison with conventional precipitation treatment, ion exchange offers the following advantages[6]:
(1) Precipitation and clarification equipment requires lots of space while ion exchange equipment is very compact
(2) Metal hydroxide sludge must be transported to a landfill licensed to handle them while ion exchange avoids the generation of sludge
(3) No economical method is currently available to recover the metal values from metal finishing solutions. Therefore, the metals cannot be recycled while ion exchange allows convenient recovery of the metals
(4) Ion exchange is a versatile process which accommodates metal ion concentration variations and reasonable changes in flow rate without deterioration in performance
Despite the advantages of ion exchange treatment, disadvantage also exists. Problems typically involved with ion exchange treatment include[4].
(1) Ion exchange has no selectivity to alkaline metals such as calcium and magnesium ions
(2) Metallic fouling (from Fe3+, Mn2+, and Cu2+, etc.) on the ion exchange media
(3) Resin fouling due to oil, grease, silt, clay, colloidal silica, organic materials, and microbes. The choice of a proper cleaning program can restore much of the lost efficiency
(4) Not all dissolved ions are removed equally; each ion exchange resin is characterized by a selectivity series, and some dissolved ions at the end of the series are only partially removed
(5) The presence of free acid reduces the efficiency of operation
(6) Fairly high operational costs and complicated operation of the system for the regeneration of resin
Biserka et al.[7] tried to kinetic analysis of the exchange processes between sodium ions from zeolite and Cd2+, Cu2+, and Ni2+ ions from aqueous solutions. Also, the study on ion-exchange equilibria of Cu2+ and Zn2+ from aqueous solutions withcommercial cation exchange resin was investigated[8]. Consequently, ion exchange may be capable of treating for high purity heavy metal solution and sequential operation. However, it requires pretreatment process to reduce suspended solid concentration in solution to prevent fouling or channeling.
2.3. Electrolytic recovery and electrodialysis
Electolytic metal recovery is one of a number of technologies capable of removing metals from process wastewater. Under certain conditions metals can be removed or plated out of solution using an electrolytic process. Metal finishers use specially designed electrolytic cells, also known as electro-winning cells, to remove metals from rinse water. The cells oxidize the metals onto a cathode. Other types of cells use electrodes and membranes to pull metals such as chromium (Cr6+) through a membrane to concentrate it for recovery. Elecrolysis takes time. The unit must be designed to allow the reaction to proceed to the extent desired. To electro-win metals from dilute rinse water a large electrode surface area is required because of the low efficiency of the process. Electroplating electrolytic recovery units are typically used in conjunction with recirculating rinse tanks to provide for long contact time. Electrolysis works best when the water does not contain particles, oil, or biological material. Particles, oil, and biological slime can coat and foul the electrodes in the cell, causing the efficiency to drop. Slime that adheres to the plates can slough off and cause discharge limits to be exceeded[6]. However, electro-winning is a highly energydependent and labor intensive process. The capital cost of an electro-winning process is extremely high and represents a significant portion of the total cost[9]. More developed technique is electrodialysis which combines electro-winning and membrane process. In the electrodialysis process, ionic components of a solution are separated through the use of semi-permeable ionselective membranes. Application of an electrical potential between the two electrodes causes an electric current to pass through the solution, which, in turn causes a migration of anions toward the positive electrode. Because of the alternate spacing of cation and anion-permeable membranes, cells of concentrated and dilute salts are formed[4]. However, this process also has problems similar with other electro-process and membrane process as follows:
(1) Chemical precipitation of salts with low solubility on the membrane surface
(2) Clogging of the membrane by the residual colloidal organic matter in wastewater treatment plant effluents
(3) Extremely high capital cost
Recently, integrated electrodialysis, electrolysis, and adsorption process was applied to recover Pb2+ and remove NO3- from aqueous solutions[10].
The use of membrane separation for water-reuse, wastewater volume reduction, and byproducts (such as valuable metals recovery) is gaining considerable attention in many industries. Membrane processes can be divided into two categories.
Ultrafiltration can be used to remove water from wastewater containing emulsified oil, reducing its volume. The water passes through the membrane and oil particles are retained. The water that goes through the membrane can meet FOG (fat, oil, and grease) discharge limitations. In some cases, depending on the nature of the contaminants, metal particles also may be excluded from the membrane and removed from the water passing through it. However, soluble metals pass through the membrane. For example, ultrafiltration is not applicable for the treatment of copper-plating rinse water, and water-soluble machine coolants can pick up zinc during use and zinc may get through the membrane. The ultrafiltration membranes need to be cleaned and back flushed regularly to operate efficiently. Over time they slowly plug up and periodically need to be replaced. Some wastewater contains particles that are the same size as the membrane pores. For example, automotive antifreeze contains silicates which apparently fit into the pores. The particles will quickly irreversibly plug the membrane. In this situation ultrafiltartion is not feasible. A 3.8 L/min ultrafiltration unit costs about $2,000. A 38.0 L/min system costs about $15,000. The 3.8 L/min system is designed to fit on a 208.2 L. The 38.0 L/min system is skid mounted and takes about 0.6 × 1.2 m floor space and is about 1.5 m height[11]. In 2007, an organic ultrafiltration membranein polyethersulfone is employed for the removal of cupric ions,complexed beforehand on poly (vinyl alcohol), from aqueoussolutions[12].
Reverse osmosis (RO) may be applied in small shops, especially those that have plating processes, but are typically used by larger facilities. RO removes soluble compounds such as sodium chloride. A concentrated reject stream is produced and must be disposed of. A very high-quality feed is required for efficient operation of a reverse osmosis unit. Membrane elements in the reverse osmosis unit can be fouled by colloidal matter in the feed system. So, Wastewater must be treated to remove solids prior to reverse osmosis of the membrane will plug quickly. Also, the removal of iron and manganese is sometimes necessary to decrease scaling potential. The pH of the feed should be adjusted to a range of 4.0 to 7.5 to inhibit scale formation. However, new generation composite membranes offer broad pH (pH 2.0 to 12.0) and temperature (up to 50 ℃) operating limits[13]. Otherwise, Gupta et al., separated inorganic and organic compounds by means of a radial flow hollow-fiber reverse osmosis module and analysis, modeling and simulation of hydrogen peroxide ultrapurification was studied by multistage reverse osmosis[14,15]. Major limitations associated with the use of membrane process include membrane fouling, limited life of the membranes, dissolution of the membrane by strong oxidizing agents, solvents and other organic compounds[11].
2.5. Evaporation / Distillation
The primary use of evaporation and distillation treatment has been product recovery, with some limited use to treat final concentrated wastewater residues to dryness. These techniques are basically end-of-the-line processes. Evaporation is simply boiling off the wastewater, leaving the contaminants behind, and reducing the waste volume. It is not the best option for the treatment of wastewater that contains organic contaminants, such as solvents, that will boil off with the water. Therefore, generally, evaporative processes are economical only for concentrated rinses and multi-stage countercurrent rinsing[16]. Metal finishers use evaporators to concentrate rinse water from certain process tanks to return it to the process tanks, recycling the process chemicals which were dragged out in the rinse water. Evaporation may be used to further concentrate the residue produced by another treatment process. For example, ion exchange regenerant water or chemical precipitation slurry may be evaporated. However, this technique also, has some disadvantages: relatively high capital costs and operational costs (particularly for vacuum systems), distillation processes are energy intensive, these systems are complex, requiring trained personnel to operate and maintain them. Alves and Coelhoso[17] compared osmotic evaporation (OE) and membrane distillation (MD) in terms of water flux and aroma retention in orange juice.
2.6. Coagulation / Flocculation
Coagulation and flocculation consist of adding a floc-forming chemical reagent to a water or wastewater to enmesh or combine with non-settleable colloidal solids and slow-settling suspended solids to produce a rapid-settling floc. Coagulation is the addition and rapid mixing of a coagulant, resulting in destabilization of the colloidal and fine suspended solids, and the initial aggregation of the destabilized particles. Coagulation is one of the most important methods for wastewater treatment, but the main objects of coagulation are only the hydrophobic colloids and suspended particles. Flocculation is the slow stirring or gentle agitation to aggregate the destabilized particles and form a rapid settling floc. This technique has been known to be capable of removing heavy metals from solution. Pang et al.[18] effectively removed lead, zinc, and iron by means of coagulation-flocculation method. Also, metal cations were removed from water by coagulation-flocculation of the chitosan-montmorillonite system [19]. Environmental Protection Agency (EPA) investigated the use of lime softening and coagulation (using ferric sulfate or alum) for removal of such heavy metals as Pb2+, Cd2+, Cr3+, and Cr6+. However, in general, coagulation-flocculation can’t treat the heavy metal wastewater completely. Therefore, coagulation-flocculation must be followed by other treatment techniques[20].
Foam flotation depends on the use of a surfactant that causes a non surface active material to become surface active, forming a product that is removed by bubbling a gas through the bulk solution to form foam. The use of foam flotation techniques for removal of heavy metals has been studied extensively[21,22]. With dilute wastewater containing heavy metals in the parts perbillion or parts per million ranges, foam flotation offers several distinct advantages. Dissolved air flotation (DAF), ion flotation and precipitation flotation are the main flotation processes for the removal of metal ions from solution. The comparative cost of foam flotation is reported to be competitive with that of lime precipitation.
2.8. Complexation / Sequestration
Complexation involves the formation of a complex compound through a complexing or chelating agent. Sequestration involves the removal of a metal ion from solution by formation of a complex ion that does not have the chemical reactions of the ion that is removed[23]. Complex formation alters the chemical characteristics of the metal ions and affects the removal mechanisms involved[24].
Cementation is a metal-replacement process in which a solution containing the dissolved metallic ions comes in contact with a more active metal such as iron[25,26]. Cementation is thus the recovery of an ionized metal from solution by spontaneous electrochemical reduction to the elemental metallic state with subsequent oxidation of a sacrificial metal (such as iron). The cementation process can be predicted in terms of electrode potentials. Advantages of the process include:
(1) Simple control requirements
(2) Low energy utilization
(3) Recovery of valuable high purity metals, such as copper
The rate of cementation was independent of the presence of oxygen. Copper cementation was independent of pH; however, above pH 3.0, ferric hydroxide precipitation masked and interfered with copper recovery. The copper from the continuous reactors had a moisture content of -38%; the dried cement contained -95.9% pure copper on a dry weight basis. A recent application of this technology involved the suspension of scrap iron in a perforated rotating drum through which the wastewater flows. Copper is cemented onto the iron and scraped off as particulate copper as it tumbles within the drum[27].
The heavy metal removal using physical and chemical processes will be continuously developed because of easiness of
[Table 1.] Physical and chemical processes for heavy metal removal[28]
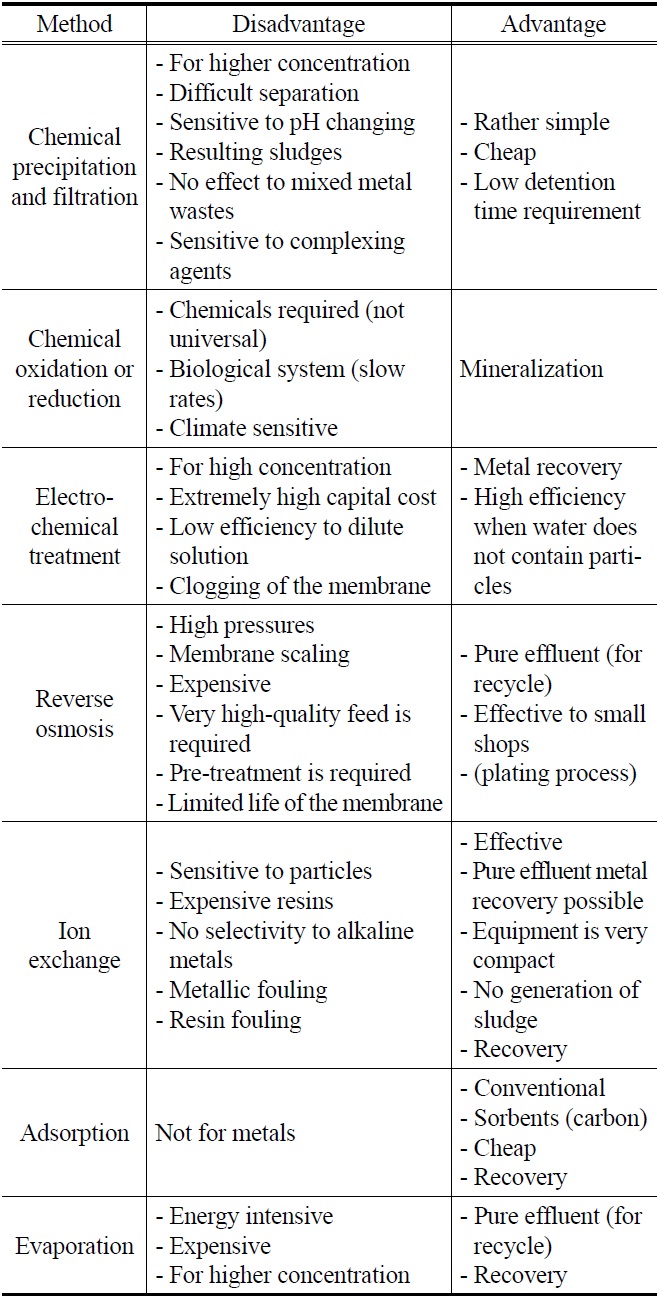
Physical and chemical processes for heavy metal removal[28]
control and high removal efficiency compared with biological treatment. Especially, interests in physical and chemical methods are sharply increasing again because of dangerousness for radioactive element. Above all, improvement of treatment efficiency for heavy metal ions will be very important to compete with other technique. Finally, the physical and chemical approaches to heavy metal removal mentioned above are summarized in Table 1.
![Physical and chemical processes for heavy metal removal[28]](http://oak.go.kr/repository/journal/11714/CJGSB2_2012_v18n4_341_t001.jpg)