



Reverse osmosis (RO) technology has developed over the past 40 years to control a 44% market share in the world desalting produc-tion capacity and an 80% share in the total number of desalination plants installed worldwide. The application of conventional and low-pressure membrane pretreatment processes to seawater RO (SWRO) desalination has undergone accelerated development over the past decade. Reliable pretreatment techniques are required for the successful operation of SWRO processes, since a major issue is mem-brane fouling associated with particulate matter/colloids, organic/inorganic compounds, and biological growth. While conventional pretreatment processes such as coagulation and granular media filtration have been widely used for SWRO, there has been an increased tendency toward the use of ultrafiltration/microfiltration (UF/MF) instead of conventional treatment techniques. The literature shows that both the conventional and the UF/MF membrane pretreatment processes have different advantages and disadvantages. This re-view suggests that, depending on the feed water quality conditions, the suitable integration of multiple pretreatment processes may be considered valid since this would utilize the benefits of each separate pretreatment.
While water scarcity occurs frequently in arid regions, pollu-tion and the use of groundwater aquifers and surface water have also led to a reduction in the quantity and/or quality of available natural water resources in many countries. Over 1 billion people are without clean drinking water, and approximately 2.3 billion people (40% of the world population) live in regions with water shortages [1]. However, the ongoing growth of population, in-dustry, and agriculture is further increasing the demand for wa-ter. In addition, higher living standards, especially in industrial countries, have resulted in higher per capita water consumption and in intensified water scarcity. Exploitation of natural fresh water resources combined with higher water demand has led to an increased demand for alternative fresh water resources. Both desalination and water reuse have been successfully incorporat-ed to provide additional fresh water production for communities using conventional water treatment and fresh water resources [2, 3]. Throughout the world, a trend towards the intensified use of desalination as a means to reduce current or future water scarcity can be observed. Seawater desalination provides such an alternative source, offering water otherwise not accessible for irrigational, industrial, and municipal use [4]. Desalination has become an important source of drinking water production, with thermal desalination and membrane processes being developed over the past 60 and 40 years, respectively [5]. In thermal de-salination, salt is separated from water by evaporation and con-densation, whereas in membrane desalination, water diffuses through a membrane, while salts are almost completely retained [6]. The decision for using a specific desalination technique is influenced by the feed water salinity, required product quality, and site-specific factors such as labor cost, available area, energy cost, and local demand for electricity.
A great share of the world’s desalination capacity is installed in the Middle East, and although reverse osmosis (RO) is rap-idly gaining the market share, thermal processes still dominate this market due to the low cost of fossil-fuel-based energy in this region and their suitability for combining with the generation of electric energy (cogeneration of steam and electricity). RO technology has been developed over the past 40 years to control a 44% market share in the world desalting production capacity and an 80% share in the total number of desalination plants in-stalled worldwide [7]. The use of RO has increased in seawater desalination over the last decade, since materials have improved and costs have decreased. Because RO membranes effectively reject monovalent ions such as NaCl, seawater RO membranes have very high salt rejections (>99%) [8, 9]. Some membranes have shown extremely high salt removal rates of as high as 99.7?99.8% when operated under standard test conditions (32,000 mg/L NaCl, pH 8, 5.5 MPa, and 8% recovery) [10].
Several limitations remain in the use of an RO membrane in seawater RO (SWRO) treatment. One of the major problems for SWRO is membrane fouling associated with particulate matter/colloids, organic/inorganic compounds, and biological growth. Suspended and colloidal particles foul a membrane by coagu-lating together and forming a cake-like layer on the membrane surface, while dissolved organics interact directly with the mem-brane surface and with each other to cause fouling [11]. Col-loidal particles are often composed of clay, organics, and metal inorganics, such as aluminum and iron silicates. Although cal-cium carbonate precipitation in SWRO is another concern, the lower SWRO recoveries (limited by osmotic pressure) prevent any precipitation problems. As a result, precipitation is unlikely to occur in SWRO applications [12]. Biological fouling associated with bacteria, fungus, or algae occurs when microbial cells accu-mulate and attach to the surfaces of a membrane and promote growth as a biofilm [13, 14]. As membrane fouling occurs, basic membrane functions deteriorate, including salt passage through the membrane, permeate flow, and pressure drop across the membrane. Inorganic scaling caused by exceeding the solubil-ity of soluble salts is considered relatively less problematic since this can be controlled by pH and adding antiscalant [15]. In ad-dition, chemical cleaning using acid and/or base is often used for RO membrane fouling [16].
It is necessary to pretreat feed water in SWRO to lower un-desirable fouling materials, since poor feed water quality leads to a short RO membrane lifetime, short operation period, and high maintenance. Pretreatment can alter the physicochemi-cal and/or biological properties of feed water and improve the performance of SWRO. Various conventional and advanced pre-treatment methods have been used in SWRO desalination. In general, conventional pretreatment includes coagulation/floc-culation, pH adjustment, scale inhibition, and media filtration. However, a new pretreatment trend has focused on the use of large pore size membranes, including microfiltration (MF) and ultrafiltration (UF), to pretreat SWRO feed water. At present, a very significant trend includes the use of membrane-based pre-treatment to improve the performance in SWRO [4, 17-25]. The objective of this paper is to review the major pretreatment pro-cesses that have been used in SWRO and to review case studies on low-pressure membrane pretreatment (UF/MF).
2. Pretreatment for SWRO Desalination
Seawater resources typically have a higher tendency for membrane fouling and require more extensive pretreatment processes than surface water and groundwater resources [7]. Therefore, a main factor for the successful operation of SWRO is maintaining a constant high feed water quality.
2.1. Conventional Pretreatment
Thus far, coagulation is the most popular treatment pro-cess used for the removal of potential foulants such as aqueous particulate and colloidal matter. The role of coagulation is to combine small particles into larger aggregates/flocs (i.e., large groups of loosely bound suspended particles) by neutralizing the charges of the particles [26]. Inorganic coagulants, including iron salts, are commonly used in the SWRO desalination plant in Madinat Yanbu Al-Sinaiyah, Yanbu Industrial City, Saudi Arabia; the plant has a capacity of 13.3 million gallons per day (MGD) [27]. In the case of this plant, in order to reduce the amount of suspended solids and colloids in the feed water, inline coagu-lation and flocculation using ferric chloride and organic poly-electrolyte are employed for pretreatment. For pretreatment in water, Al and Fe salts are probably the most commonly used coagulants; they first react with water to form a series of cat-ionic hydrolytic species and weakly charged or uncharged pre-cipitates [28]. However, for SWRO, aluminum is not as frequently used as a pretreatment coagulant prior to membrane filtration due to potential damage to the membrane system. Typically, a wide range of inorganic coagulants (5?30 mg/L) is used, while a significantly smaller dose is required for a polymer coagulant (0.2?1 mg/L) [29].
When the feed seawater quality becomes relatively less poor and does not require the full process of flocculation and sedi-mentation, inline coagulation can be used prior to media filtra-tion. In this process, the coagulated water is directly introduced to the membrane filtration system. Inline coagulation that changes the surface chemistry of the suspended particles en-hances their attachment to the media filter. Inline coagulation in the absence of flocculation/sedimentation can reduce the foot-print of the entire membrane filtration facility [30]. In a full-scale SWRO plant, flocculants (ferric chloride sulfate) are added to the untreated water at the inlet to the destabilization tank [21]. In the study, an acid dosing system was used to prevent carbon-ate scaling, a polyelectrolyte dosing system and three in-line co-agulation filters with ferric chloride sulfate were used to further reduce the silt density index (SDI) of the feed to less than 3.0, so-dium hydrogen sulfite dosing was used to remove residual chlo-rine, and three cartridge filters were used to remove particles larger than 5 μm. Although the pretreatment with coagulation significantly enhances the removal of colloidal and particulate matters, a previous study showed that coagulant residuals from the pretreatment process may negatively affect RO membrane performance when either aluminum/iron salts or chloramines are used [31]. In that study, both the specific flux (up to 60%) and salt rejection were significantly reduced when alum was used with multiple RO element testing for over 100 hr of operation.
SWRO membranes can be subject to salt precipitation and membrane scaling. Precipitation has been widely investigated in the RO process between two bench-scale brackish water RO units that increased the water recovery from the typical 90-98% overall [32, 33]. The precipitation process consisted of using either calcium carbonate (calcite) or calcium sulfate seeding, along with pH control, to remove slightly soluble salts. While gypsum seeding achieved a calcium removal of only 30%, calcite seeding achieved 92?93% calcium removal within 30 min [33]. In SWRO treatment, one of the most challenging issues is to remove boron. Boron has adverse reproductive and development effects and causes plant and crop damage [34]; it is difficult to remove by RO membranes, since it naturally exists as a non-ionic species due to a relatively high pKa (pKa = 9.2 for fresh water and 8.5 for seawater) in seawater within the concentration range of 4.5?6.0 mg/L [12, 35]. Boron rejection can be increased by increasing the feed water pH. However, increasing the pH can cause salt pre-cipitation and subsequent membrane scaling (i.e., deposition of salt precipitates on the RO membrane). Therefore, multiple RO stages are often required to enhance boron removal at differ-ent pH conditions, where the first stage (at lower pH) achieves salt removal and the second stage (at higher pH) achieves boron removal [36-39]. pH adjustment can effectively control calcium carbonate scaling, while scale inhibitors using antiscalants have been used to control various carbonate, magnesium hydroxide, sulfate, and calcium scaling [20].
Conventional packed-bed filters using granular media such as sand, anthracite, pumice, gravel, and garnet with different effective sizes are beneficial in terms of regeneration, since hy-draulic backwashing has proven to be effective in conventional water treatment in restoring capacity [18, 40, 41]. For constant physicochemical conditions, the granular media filtration pro-cess is effective at removing particles significantly larger than a few micrometers or smaller than 0.1 μm [42]. As water pass-es through the filter bed, the suspended particles contact and adsorb onto the surface of the individual media grains or onto previously deposited material [20]. To achieve a high treated water quality, the surface charge, size, and geometry of both suspended particles and filter media are major parameters. The U.S. Army Water Desalination Technical Manual suggests effec-tive grain sizes of 0.35?0.5 and 0.7?0.8 mm for fine sand and an-thracite filters, respectively [43]. The turbidity of media filtrate is often around 0.1 NTU [2, 18]. The media filtration SDI can be sensitive to feed water changes containing algal blooms and oil contamination. In particular, oil contamination is a difficult problem and is most often removed using dissolved air flotation (DAF) during membrane pretreatment [44]. In addition to oils, DAF is a commonly utilized process for removing a number of pollutants, including colloids, fine and ultra-fine particles, pre-cipitates, ions, microorganisms, and proteins [45]. In this study, compared to the typical sedimentation process, DAF allows light particles that settle slowly to be removed more effectively and in a shorter time; it also usually produces a low generation of sludge from the system. The RO feed water was maintained to be less than 0.25 NTU and had an SDI of less than 1.5 on av-erage with the coagulation (ferric chloride) and DAF pretreat-ment processes raw seawater quality is characterized by a high conductivity level (37,900?52,000 μS/cm), total dissolved solids (TDS) in the range of approximately 25,000?50,000 mg/L, pH 8?8.5, and turbidity in the range of 5?20 NTU [18].
As previously described, the conventional pretreatment pro-cess has been widely used for SWRO. However, since it needs to be carefully designed and diligently operated, there has been an increased tendency toward using UF/MF instead of the con-ventional treatment to provide SDI values well below 2, which thus enables an SWRO plant to perform at its original design capacity with reduced downtime [46]. Frequently, colloids and suspended particles that pass through conventional pretreat-ment contribute to difficult-to-remove (and possibly irrevers-ible) RO membrane fouling [8]. Therefore, the use of larger pore size membranes such as UF and MF has gradually gained ac-ceptance in recent years as the preferred pretreatment for SWRO [47]. Pilot and/or full-scale plants have been operated in many parts of the world to examine the capacity and reliability of UF/MF pretreatment systems in preparing compatible feed water for the SWRO membrane [8, 19, 20, 22]. In addition, successful implementation of nanofiltration (NF) pretreatment has been conducted for the RO process [48-50]. Among the NF, UF, and MF membranes, UF membranes seem to be the most common preference in research studies and pilot testing [8, 19, 25, 29, 47, 51-53] and represent perhaps the best balance between contam-inant removal and permeate production of the three membrane types; UF membranes have smaller pore sizes than MF mem-branes and higher flux than NF membranes. However, each membrane can be selected depending on the specific contami-nant removal issues, since they have different advantages. For instance, MF membranes are the appropriate choice for removal of larger particulate matter at higher permeate fluxes, whereas NF membranes are used to remove dissolved contaminants as well as particulate and colloidal material [7].
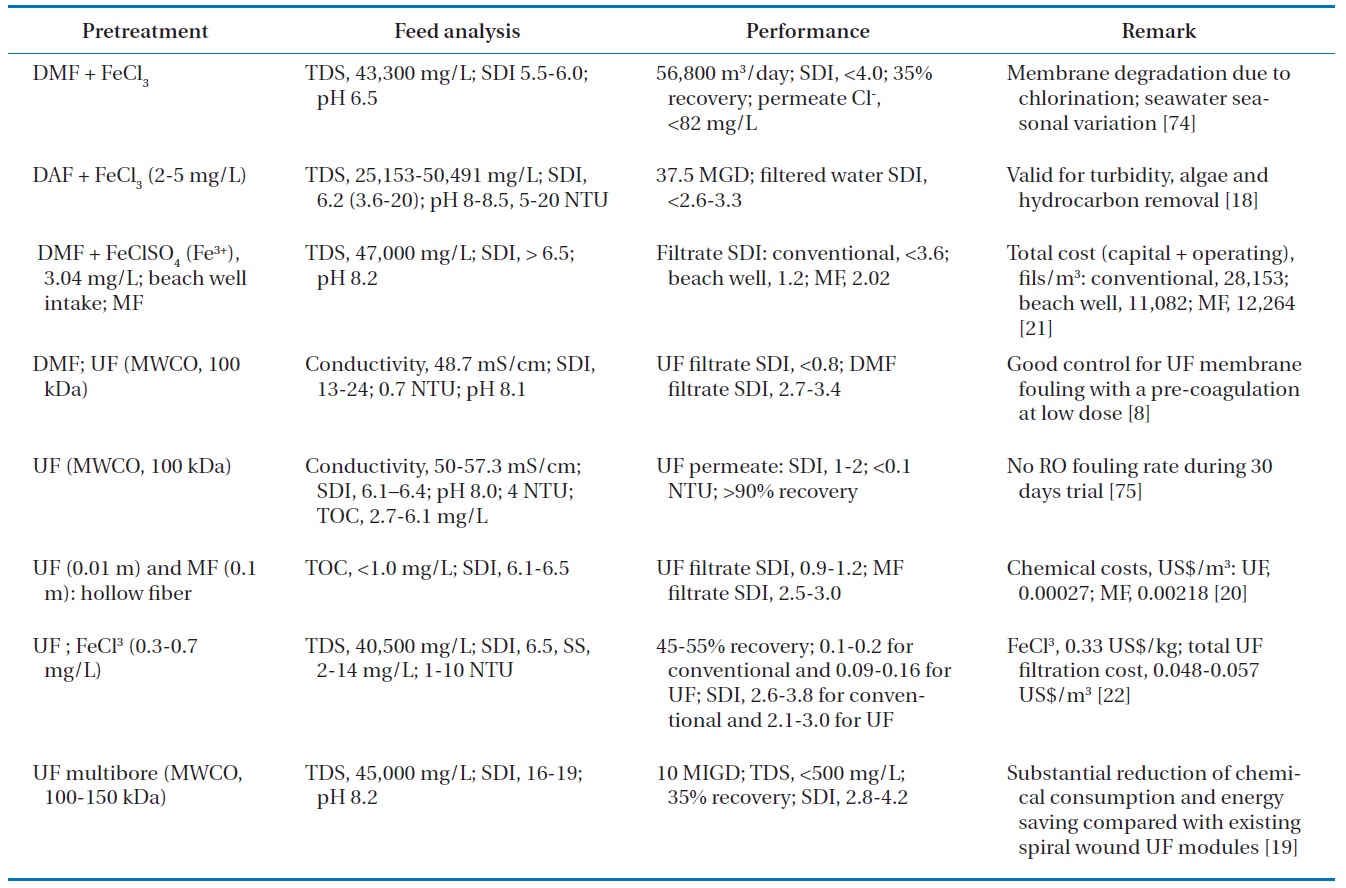
Several pilot and/or full studies using a membrane pretreat-ment system have been conducted with various seawater quality conditions, different low pressure membranes, and different op-erating conditions. Gulf seawater with high salinity and bioactiv-ity was treated at the SWRO plant in Bahrain; the plant consisted of a prechlorination unit, sand filtration, and UF membranes (20 nm pore diameter, molecular weight cutoffs of 100,000?150,000, average flux of 70 million liters per hour, filtration time of 17?20 min, and chemical-enhanced backwash) [19]. Stable operation at a constant flux was achieved during the summer months. The Multibore membranes (Inge AG, Greifenberg, Germany) allow for a substantial reduction in chemical and energy consumption compared with existing spiral-wound UF modules. In a separate study, a field testing program was conducted at Ashdod on the Mediterranean to compare performances of RO seawater sys-tems operating on the surface seawater using conventional pre-treatment and the UF membrane technology [22]. The Mediter-ranean Sea has turbidity in the range of 1?10 NTU, a TDS value of 40,500 mg/L, an SDI consistently above 6.5, and suspended solids in the range of 2?14 mg/L. The SDI was reduced to 2.6?3.8 for the conventional system and 2.1?3.0 for the UF membrane system. In addition, in spite of the fluctuating seawater quality, the filtrate produced by the UF system could still be accepted by the RO membrane system. Another study was conducted over four months on a pilot plant platform installed at the desalina-tion plant of ONDEO Services in Gibraltar [8]. The seawater at Gibraltar is known to be difficult because it is subject to algae blooms twice a year and is characterized by a conductivity of 48.7 mS/cm at 20℃ and an SDI of between 13 and 15. The study first showed that the removal of fouling constituents of seawa-ter was more efficient with UF pretreatment than with conven-tional pretreatment: UF reduced SDI from 13?25 to less than 0.8, whereas with the dual media filter, the filtrate SDI remained between 2.7 and 3.4. The UF permeate had a constant quality for the entire duration of the experiment, whereas the quality of the dual media filter (DMF) filtrate fluctuated significantly with respect to turbidity.
While UF and MF membranes are more highly preferred than NF membranes, they may still need pretreatment processes in-cluding coagulation, adsorption, oxidation, and media filtration to reduce membrane fouling and/or increase the removal of cer-tain aquatic contaminants. Major mechanisms and the effects of these pretreatments are summarized in Table 1 together with the advantages and disadvantages of these pretreatments. The effects of coagulant dose on membrane fouling are related to the properties of coagulants [54-56] and the type of UF and MF membranes [55, 57]. Although the cost-effective coagulant doses reported in the literature for membrane fouling reduction can differ from the optimal doses for conventional water treatment, bench-scale or pilot-scale tests are often necessary in order to determine the effects of coagulant dose on a particular source water and membrane of interest [40]. As briefly summarized previously, coagulation pretreatment can significantly improve low-pressure membrane performance (less fouling and greater rejection), while it 1) requires a proper dose that can be difficult to meet if feed water quality varies rapidly/significantly, 2) may exacerbate fouling, 3) produces solid wastes, and 4) may be inef-fective in mitigating the fouling by hydrophilic neutral organics (Table 1).
Absorbents are favorable to UF and MF membranes as they are poor at removing the small substances [58]. The most inten-sively studied adsorbent for UF/MF filtration is powdered ac-tivated carbon (PAC). The efficacy of PAC in removing organic contaminants is strongly dependent on the PAC type [59, 60], dose and properties of the organics [61, 62], and the competi-tion of other aquatic constituents [63]; PAC may also remove in-organic contaminants, such as arsenic [64]. Granular activated carbon (GAC) filters have been integrated with low-pressure
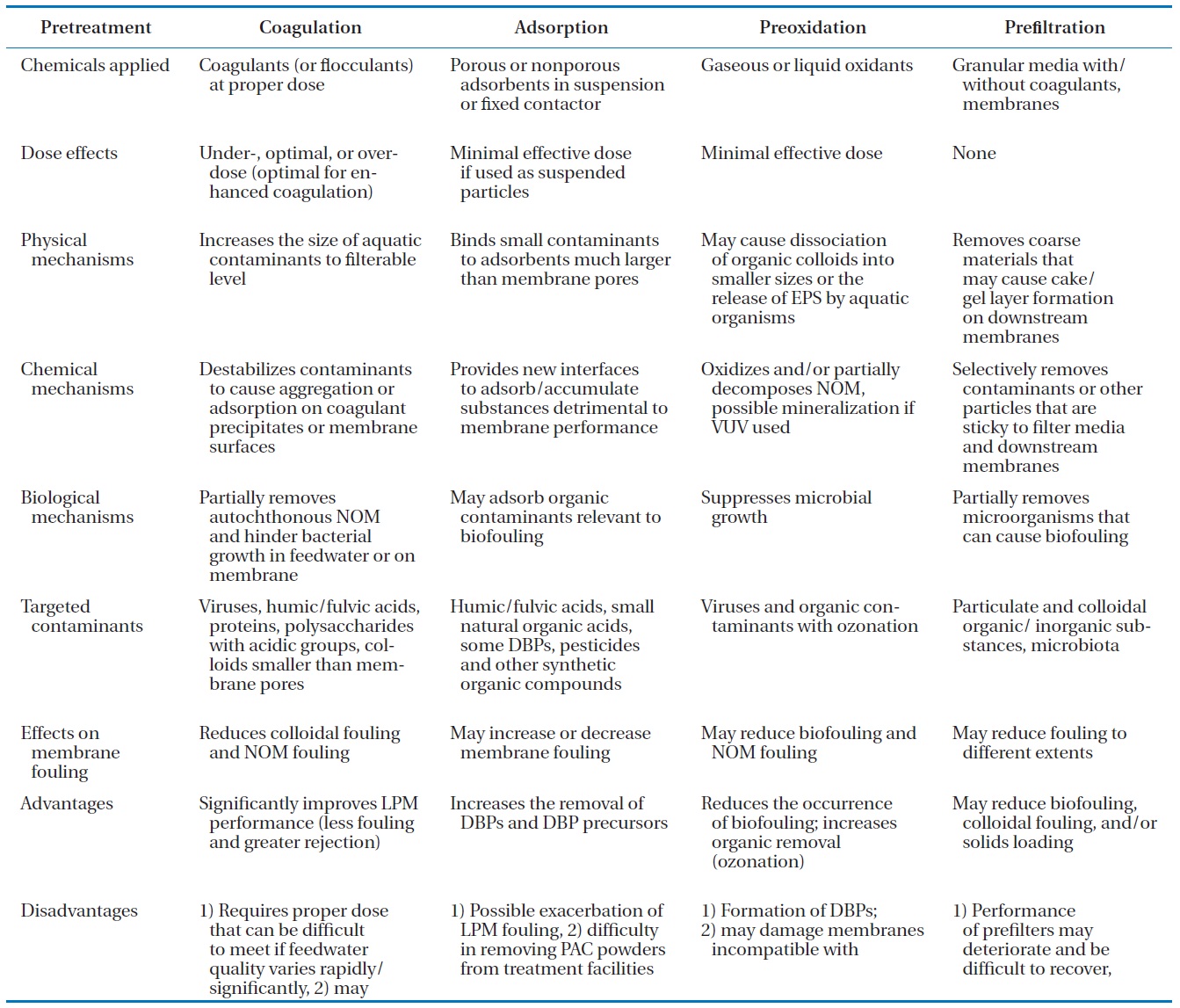
List of the mechanisms effects and applications of major pretreatments for membrane filtration [40]
membrane filtration in pilot-scale testing [65, 66]. These studies found that GAC prefiltration/adsorption effectively reduced the irreversible fouling of some UF membranes in treating natural surface water. Recently, carbon nanotubes (CNTs) have drawn special research attention due to their unique properties and potential environmental applications: sorbents, high-flux mem-branes, depth filters, antimicrobial agents, environmental sen-sors, renewable energy technologies, and pollution prevention strategies [67, 68]. In addition, CNT technology has the potential to support point-of-use in water treatment since, unlike many microporous adsorbents, CNTs possess a fibrous shape with a high aspect ratio, a large accessible external surface area, and well-developed mesopores, all of which contribute to the supe-rior removal capacities of these macromolecular biomolecules and microorganisms [69]. Due to these unique characteristics of CNTs, the potential applications of CNT-UF/MF can have considerable benefits in water/wastewater treatment/reclama-tion and seawater desalination, although they have not been studied. Adsorption is advantageous to combat the increase in disinfection byproducts (DBPs) and DBP precursors as well as membrane foulants. However, adsorbents can possibly exacer-bate membrane fouling and be difficult to remove from treat-ment facilities (Table 1).
Seawater containing microorganisms such as bacteria, al-gae, fungi, and viruses can cause serious biological fouling. This biofouling can be controlled by chemical oxidants (chlorine, bromine, iodine, or ozone), ultraviolet irradiation, biofiltration to remove nutrients, and the addition of biocide [4]. At oxidant doses practical for pretreatment, previous studies of conven-tional water treatment have demonstrated that chlorine and permanganate can be added to the feed water to suppress the growth of microorganisms and maintain oxidative conditions in the water; ozone can partially oxidize natural organic matter (NOM) and increase the assimilable organic carbon that may be removed by downstream biological filters [70]. A previous study found that the concentration of manganese in the permeate of a pilot-scale UF system decreased from 0.16?0.19 mg/L to below the targeted 0.05 mg/L with the addition of KMnO4 as a result of oxidation and precipitation of soluble Mn species [71]. The presence of low levels of ozone may also improve the removal of organic or organic-coated particles by coagulation and filtra-tion, which indicates a change to the stability or reactivity of
[Table 2.] Detailed case study analysis of conventional and UF/MF pretreatments for SWRO
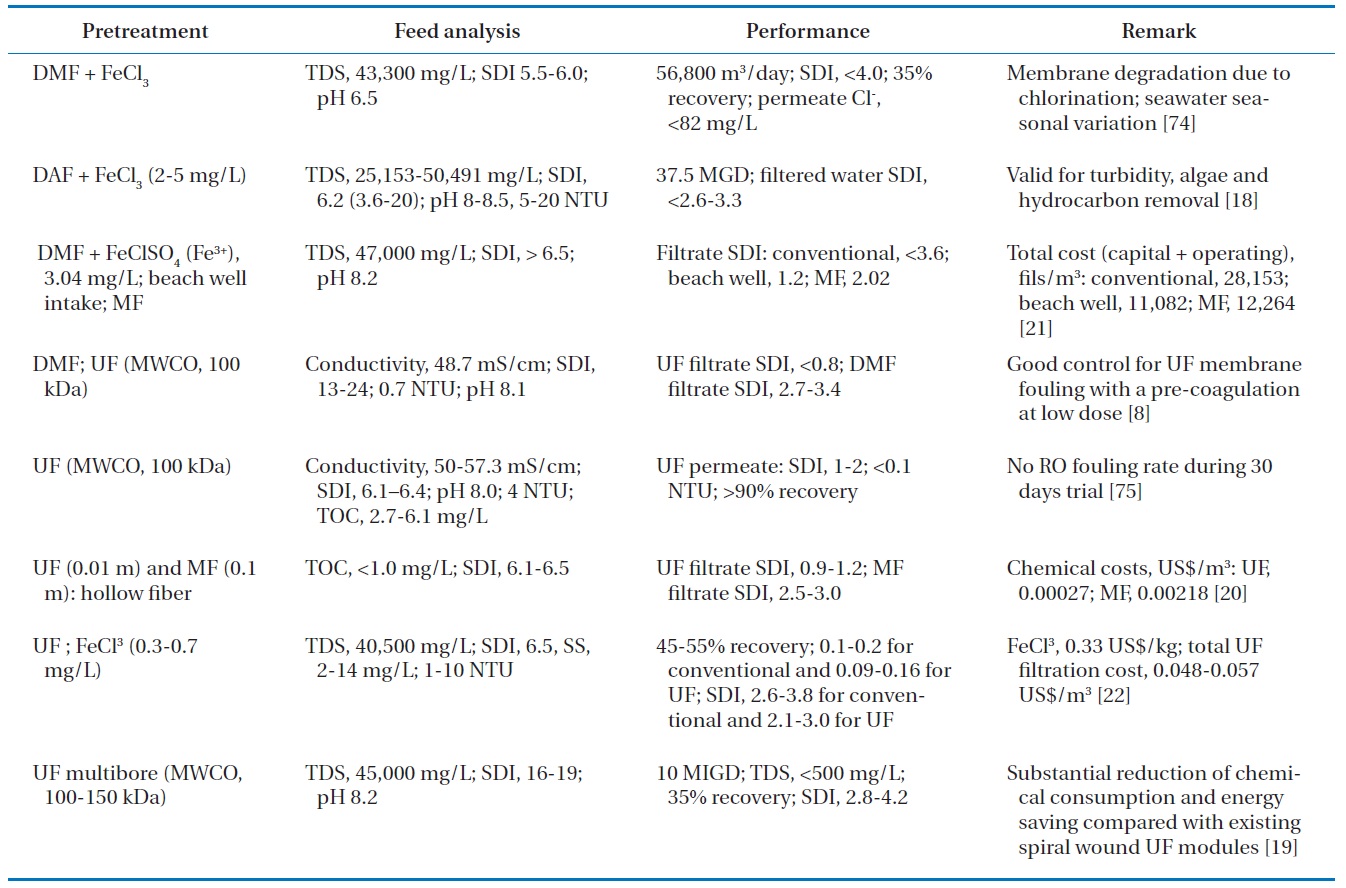
Detailed case study analysis of conventional and UF/MF pretreatments for SWRO
aquatic particles with respect to coagulation or deposition [72, 73]. While preoxidation is valid for reducing the occurrence of biofouling and NOM removal, it has several disadvantages such as the formation of DBPs, membrane damage, and ineffective-ness in suppressing the growth of some microbiota resistant to oxidation [40]. Table 2 summarizes a more detailed case study analysis of conventional and UF/MF pretreatment for SWRO with various feed waters and operating conditions.
Different pretreatment technologies often preferentially re-move certain types of aquatic contaminants or have different ef-fects on SWRO membrane fouling. Therefore, depending on the feed water quality conditions, it may be necessary to consider the proper integration of multiple pretreatments and combine the benefits of each separate pretreatment. Raw water with ag-gressive and fluctuating chemistry quality presents a challeng-ing task for designers in selecting the appropriate pretreatment technology for fending off design deficiencies at a later stage [46]. The conventional treatment scheme may not work in ev-ery scenario and may not always be the right choice. In terms of an economic point of view, although the integration of multiple pretreatments may increase the capital costs of the system, the
[Table 3.] Cost analysis comparison of conventional and UF/MF pretreatments [46]
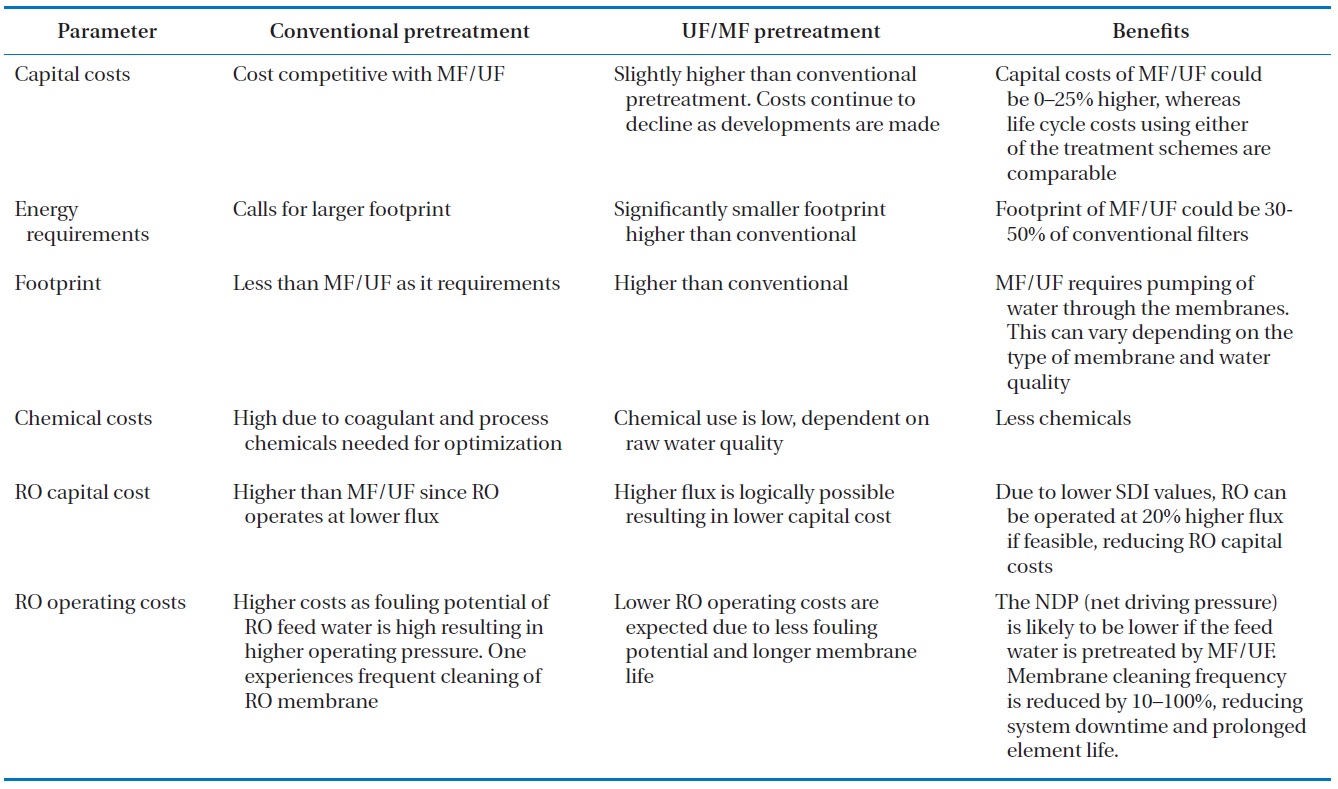
Cost analysis comparison of conventional and UF/MF pretreatments [46]
operational costs may decrease if membrane fouling can be ef-fectively reduced by the integration. Table 3 shows a cost analy-sis between the application of conventional pretreatment and the UF/MF pretreatment [46]. This overview indicates that it is feasible to combine UF/MF with RO, which is a well-established technique for water desalination and reuse in the Middle-East-ern states. In addition, lower chemical cleaning frequency and RO membrane replacement are expected due to the superior UF/MF permeate water quality as well as the benefits of a re-duced footprint and easier operation of UF/MF [4].
![List of the mechanisms effects and applications of major pretreatments for membrane filtration [40]](http://oak.go.kr/repository/journal/11630/E1HGBK_2011_v16n4_205_t001.jpg)
![Cost analysis comparison of conventional and UF/MF pretreatments [46]](http://oak.go.kr/repository/journal/11630/E1HGBK_2011_v16n4_205_t003.jpg)