Boron is an essential element for both animals and humans. Boron plays a role in human brain function, affects perception and temporary memory [1] and has protective characteristics against prostate cancer [2]. However, boron can become toxic in amountsslightly greater than required. Some symptoms of boron poisoning in humans are nausea, vomiting, diarrhea, dermatitis and lethargy [3]. In 1990, World Health Organization (WHO) rec-ommended the maximum concentration limit of boron as low as 0.3 mg/L for drinking water.Complying with this standard is difficult due to the available water treatment technology. Hence, WHO raised this guideline value to 0.5 mg/L in 1998.The aver-age concentration of boron in freshwater is less than 0.1 mg/L and below 2 mg/L in groundwater [4]. In seawater, boron exists at around 4.5 mg/L [5].
Fresh water is an invaluable resource. People use it for drink-ing, cooking, washing, producing materials, etc.Nearly 97% of all water in the world is salty or otherwise undrinkable. Another 2% is locked in ice caps and glaciers, with only 1% remainingfor all of our needs [6]. There are many scarcity areas in the world and the water source of local people depends largely on desalination plants. Desalination is the process that removes a certain amount of salt and other minerals from the water.Reverse osmosis (RO) is the major membrane technology for seawater desalination. It is found that, with one pass SWRO (seawater RO), boron removal only reaches 0.8-1.0 mg/L in permeatefor an initial boron con-centration in seawater feed of 4.0-5.0 mg/L. This does not com-ply with the WHO standard for boron concentration in drinking water. In order to reduce boron concentration in RO permeate and comply with the standard requirement, several designs have been developed such as the second pass SWRO with increasing pH and the second pass SWRO with boron selective resin or Cas-cade design (IDE's patented design process) [7].
Coagulation and flocculation are important phenomena in water and wastewater treatment. Coagulation in water treatment is a process of combining small particles into larger aggregates for settling. Common coagulants which are used for water treat-ment are classified into metal coagulants and polymers. There are two general categories of metal coagulants: those based on aluminum and those based on iron.Polymer coagulants can be natural or synthetic, and water-soluble or macro-molecular [8].
In this study, a coagulant called Mineral Cluster was exam-ined for boron removal. The objective of this study was to inves-tigate the boron removal from aqueous solution using Mineral Cluster. The pH value of the solution and the Mineral Cluster dosage were tested to determine the most suitable condition for eliminating boron.
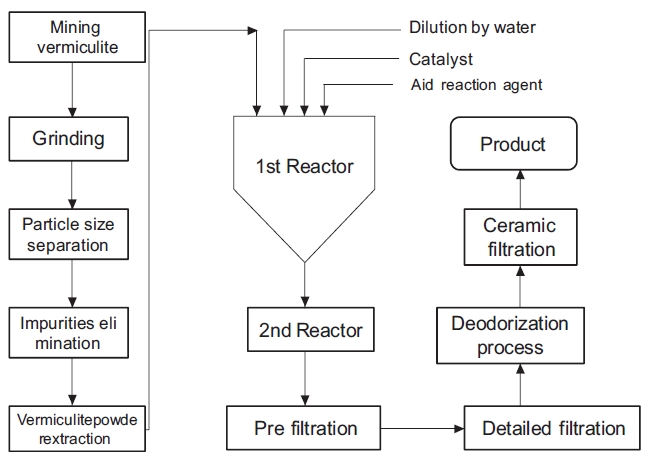
The coagulant used in the experiment is called Mineral Clus-ter. Mineral Cluster is a product extracted from vermiculite. The procedure for producing Mineral Cluster is shown in Fig. 1. Min-eral Cluster solution has a yellow-green color and contains seven
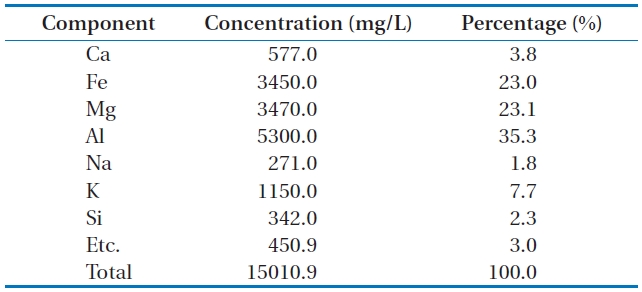
main components:calcium (Ca), iron (Fe), magnesium (Mg), alu-minum (Al), sodium (Na), potassium (K), and silicon (Si). Table 1 lists the main components of Mineral Cluster and their propor-tions.
Aluminum component has the largest proportion in the Min-eral Cluster composition with 35.3% of weight. Iron and magne-sium rank second with proportionsof 23.0% and 23.1% of weight, respectively. Al, Fe, and Mgare important elements in the compo-sition of the common coagulants.
A spiral wound RO system was utilized to evaluate boron re-moval ability in aseawater desalination process in which Min-eral Cluster was used. Microfiltration and ultra-filtration are pre-treatments for this RO system.
All samples utilized in the experiment were made from dis-tilled water. Mineral Cluster solution was diluted with the follow-ing ratios; 1:1, 1:10, 1:20, and 1:100. The bulk water was stored in 100 mL beakers as follows:
1) “1:1 Mineral Cluster” (sample 1): 50 mL Mineral Cluster solu-tion and 50 mL distilled water.
2) “1:10 Mineral Cluster” (sample 2): 10 mL Mineral Cluster solu-tion and 90 mL distilled water.
3) “1:20 Mineral Cluster” (sample 3): 5mL Mineral Cluster solu-tion and 95 mL distilled water.
4) “1:100 Mineral Cluster” (sample 4): 1mL Mineral Cluster and 99 mL distilled water.
Boron was added to all samples so that they contained 4.5 mg/L of boron.
[Table 1.] Main components of Mineral Cluster
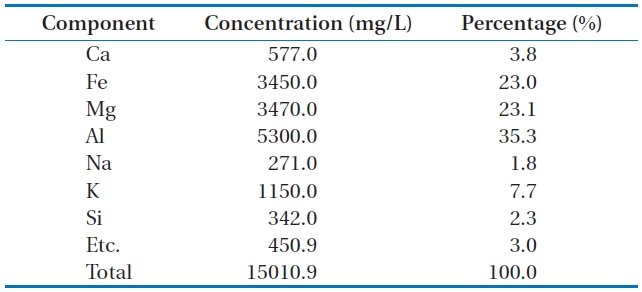
Main components of Mineral Cluster
The 1:20 Mineral Cluster was chosen to evaluate boron re-moval efficiency with changes of pH. The pH of bulk water was adjusted to the values of 7, 8, 9, and 10 by NaOH and H2SO4.
The bulk water was stirred rapidly for 2 min (to distribute the coagulant homogeneously throughout the water), followed by a slow stirring period of 30 min (to encourage flocculation), and was allowed to settle for 60 min at room temperature, and wasthen filtered by 0.45μm membranes.
To estimate the boron removal ability of Mineral Cluster in the desalination process, a pilot experiment with a spiral wound RO system was utilized and the final boron removal efficiency was calculatedby combining the efficiency of Mineral Cluster and the efficiency of the RO system.
Boron concentration was analyzed by the curcumin method [9]. This method is applicable in the 0.1-1.0 mg B/L range, so the samples were diluted before analyzing. The analysis procedure was conducted as follows:
1) Pipet1mL of the diluted samples into evaporating dishes.
2) Add 4 mLcurcumin reagent to each and swirl gently to mix the contents thoroughly.
3) Float dishes in a water bath set at 55℃ for 80 min for complete drying.
4) After dishes cool to room temperature, add 10 mL 95% ethyl alcohol to each dish and stir gently until complete dissolution of the red-colored product.
5) Wash contents of dishes in 25 mL volumetric flasks using 95% ethyl alcohol and mix thoroughly. For the samplesin which Mineral Cluster was used, their contents of Mg, Fe, and Al were rather high, and they formed white scales after wash-ing with ethyl alcohol; therefore, it is necessary to filter the samples with filter paper after washing with ethyl alcohol for removing interference.
6) Read the absorbance of the samples at a wavelength of 540 nm after setting the reagent blank at zero absorbance.
7) We can calculate the boron concentration based on the cali-bration curve made previously.
The boron removal efficiency was calculated by the following formula:
R : boron removal efficiency (%)
Cin : initial boron concentration (mg / L)
Cout : final boron concentration (mg / L)
3.1. Effect of Mineral Cluster Coagulant
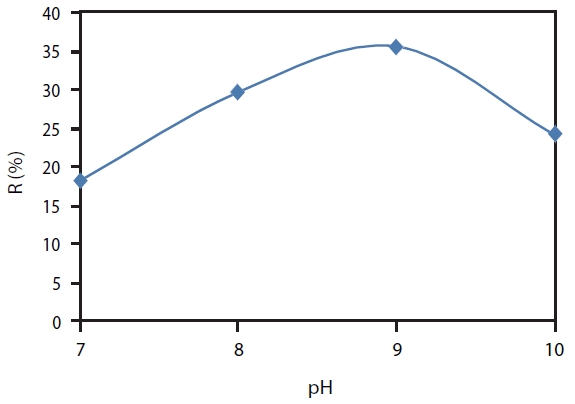
When pH is lower than 7, boron mainly exists in the un-dissociated form as H3BO3, and when pH is higher than 11, acid boric dissociates completely into the borate ion form [B(OH)4-] [10]. Therefore, boron removal depends largely on pH, and the experiment with different pH values to determine the optimum pH was necessary. The samples which contained 4.5 mg B/L and 1:20 Mineral Cluster were adjusted to pH 7, 8, 9, and 10.
The experimental result in Fig. 2 shows that at pH 7, the Min-eral Cluster coagulant only removed boron to 18.2%, and the bo-ron removal efficiency increased at pH 8 to a value of 29.7%. The highest efficiency was 35.6% at pH 9 and it reduced to 24.1% at pH 10.
This result can be explained based on the coagulation mecha-nism and boron adsorption mechanism. Alum coagulants work better for a pH range of 5.5 to 8.0, whereas iron coagulants can be used successfully in a pH range from 5.0 to 11.0 [11].In Min-eral Cluster solution, the contents of aluminum and iron were highest; they also played a main role for coagulation phenom-ena. Therefore, pH 9 was optimum for both aluminum and iron as well as for Mineral Cluster. Following the boron adsorption mechanism of Liu et al. [12],there are three types of interactions:electrostatic interaction, hydrogen bonding and hydrophobic interaction. From the physicochemical natures of boric acid, borate, and the particle surfaces, it could be deduced that hy-drogen bonding and hydrophobic interactions may play roles in the adsorption. All the particles have the suitable functional groups and atoms on their surfaces for the formation of hydro-gen bonds with boric acid or borate (including ionic hydrogen bonds), which can promote the adsorption. Hydrophobic inter-actions represent a tendency of non-polar groups to associate in aqueous solutions. At pH 9, boron existed in two forms, acid boric and borate ion, with equal quantity, and the adsorption of boron was enhanced by hydrogen bonding, and electrostatic and hydrophobic attractions. At pH larger than 9, the adsorption reduced due to lack of hydrophobic interaction. However, at pH smaller than 9, the adsorption amount of boron was smaller than that in pH 9 solution, probably because of the lack of electro-static attraction. As a result, pH 9 was the optimum pH for boron removal by Mineral Cluster.
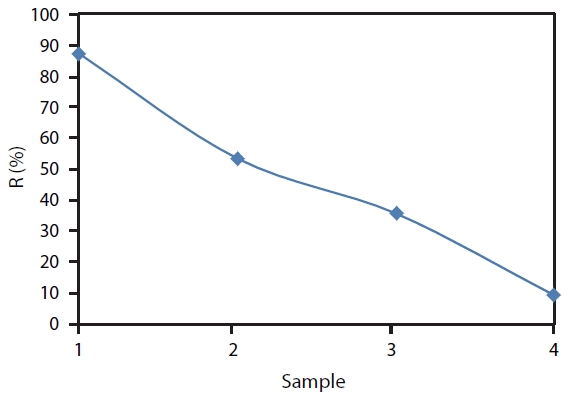
Furthermore, when increasing the Mineral Cluster dosage, boron removal efficiency was more clearly improved. The experi-ment was conducted at optimum pH 9(Fig. 3).
Obviously, as the coagulant dosage increased, the boron re-moval efficiency increased. The highest boron removal efficien-cy was at 1:1 dilution with 87.4%.The efficiency of 1:10 dilution was 53.4%. The boron removal efficiency with 1:20 dilution was 35.6% and it was 9.1% when diluting to 1:100 ratio.
When the Mineral Cluster content in solution was high, the contents of the ions in the Mineral Cluster solution were also high; the ions played an important role in neutralizing and floc-culating boron and removing it from the filtered solution.
Boric acid has a pKa of 9.14?9.25 and it is not ionized in natu-ral seawater with pH 7.0?8.0 [13]. The passage of boric acid in seawater through RO membranes is affected mostly by size ex-clusion and hindrance (molecular friction). However, the effect of hindrance is not strong because the B(OH)3 molecule is small. Another factor causing the high passage of boric acid through RO membranes could be the hydrogen bonding between its three hydroxyl groups and the bound water in the membrane (hydro-gen bridges), which enhances the association and drag of B(OH)3 by water [14].
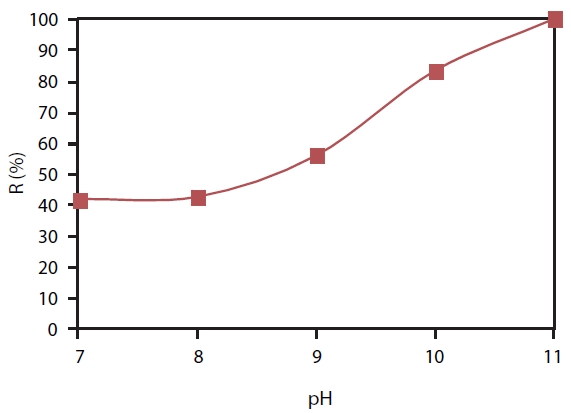
The experiment with the RO system was conducted with dif-ferent pH values. There was a clearly rising trend of boron remov-al efficiency with increasing pH. At a pH value of 7 or 8, boric acid was not significantly dissociated into the borate ion; therefore, a large amount of the boron passed through the RO membrane, and boron removal efficiency was only around 43%. When in-creasing pH to 9 and 10, boric acid dissociation was large, and the boron retention ability of RO was thereforebetter;the boron removal efficiency reached 56.0% at pH 9 and 83.4% at pH 10. The efficiency of boron removal reached 100% with pH 11 by the RO system when acid boric was dissociated completely (Fig. 4).
3.3. Calculating Boron Removal Efficiency When Com-bining Mineral Cluster Coagulant with RO System
From the above results, boron removal by RO membrane can be considered a function of pH. Using that removal efficiency, the final boron concentration as well as the final boron removal efficiency can be calculated when combining the Mineral Clus-ter coagulant and RO system. Calculation was performed with “1:20 Mineral Cluster.”
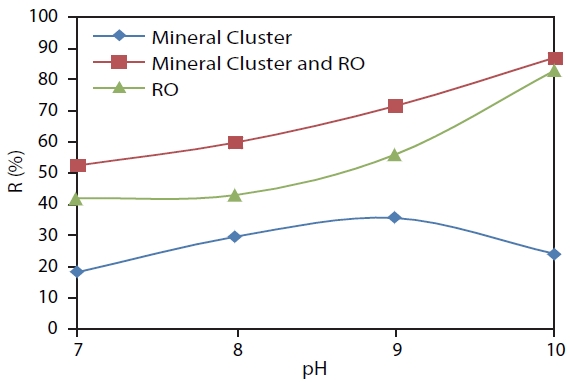
Fig. 5 shows the different boron removal efficiencieswhen using Mineral Cluster coagulantfortheRO system and Mineral Cluster combined RO system. In the three methods, the efficien-cy of the Mineral Cluster combined RO system was highest with R 52.4%, 59.8%, 71.5%, and 87.1%, respectively corresponding to pH 7, 8, 9, and 10.
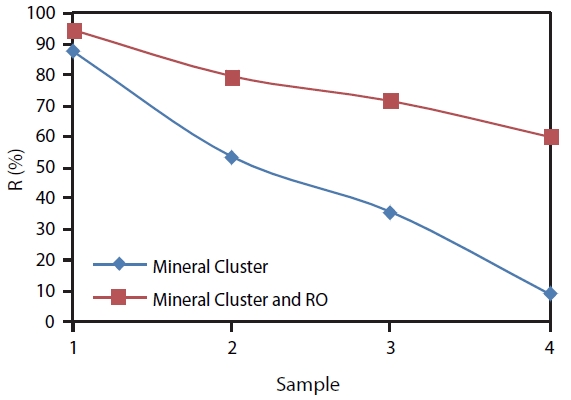
The boron removal efficiency when changing Mineral Cluster dosages in the system combining Mineral Cluster coagulant and RO was also calculated. Calculation was performed at optimum pH 9. Calculation results showed that the combination of Miner-al Cluster coagulant and RO has the highest boron removal with 94.4% at 1:1 dilution ratio; 79.5% at 1:10 dilution ratio; 71.5% at 1:20 dilution ratio; and 59.9% at 1:100 dilution ratio (Fig. 6).
Boron removal depended largely on pH because in low and neutral pH conditions, boron exists mainly in the form of un-charged boric acid and it is thereforedifficult to remove by com-mon water treatment methods.
Boron removal by RO was a function of pH. At pH 11, the RO system can remove boron up to 100%.
pH value 9 was optimum for boron removal by Mineral Clus-ter coagulant. At this pH value, boron can be eliminated up to 87.4% with 1:1 Mineral Cluster. However, at a lower concentra-tion of Mineral Cluster, the 1:20 Mineral Cluster combined RO system can remove boron up to 71.5% at pH 9, whereas the re-moval efficiency of RO wasonly 56.0%. In practice, this removal can only be reached after passing the second RO membrane or adjusting the pH; it will thereforerequire an expensive capital cost. As a result, Mineral Cluster coagulant was suitable for the RO desalination system as a pretreatment.