A scaled-up bioconversion of fishmeal wastewater (FMW) into liquid fertilizer was performed five times in a 1 m3 reactor in order to examine the feasibility of commercialization. The importance of aeration was marked. Analyses indicated that dissolved oxygen (DO) level was closely related to the value of oxidation-reduction potential (ORP) and it was crucial to achieve high-quality liquid fertilizer. When pure oxygen was supplied through four diffusers into the reactor, DO levels and ORP values were maintained over 1.2 mg/L and 0.2 mV, respectively all the time during 52 hr of bioconversion. The pH changed from 6.8 to 5.9. The average removal percentages of chemical oxygen demand (CODCr) and total nitrogen (TN) were 75.0% and 71.6%, respectively. Compared to the result acquired in a 5-L reactor, bioconversion of FMW into liquid fertilizer was achieved in a shorter time under the same removal percentages of CODCr and TN. The 52-hr culture of inoculated FMW was phytotoxic-free and it possessed comparable fertilizing ability to a liquid fertilizer made from the fish waste in hydroponic culture with amino acid contents of 5.93 g/ 100 g sample. From all the above results, transferring labscale data to large-scale production appeared to be successful. As a result, the commercialization of a liquid fertilizer made from FMW was feasible.
There is a steady increase in the worldwide fish consumption. Moreover, seafood is gaining popularity due to its health benefits. In the recent years, this trend has been reflected in Korea. Large amounts of fishery wastes are generated and they are mostly from industrial processing of fish. Accordingly, the disposal of fishery wastes greatly affects the local environment. For this reason, various types of reutilization of fishery wastes have been reported: Production of protein meals for animal feeds by ensilation [1]; fishery waste disposal by composting [2]; production of fish protein for animal feeds by fermentation [1, 3]; processing of fish waste by low-cost fermentation using
Biological treatment technologies of fish-processing wastewater have been studied to improve the effluent quality [14, 15]. A common feature of the fish-processing wastewater is their diluted protein content. This has been recovered and used in animal feed, human food, seasoning, etc. [16]. Although reutilization of this wastewater has been limited due to its bad smell [17], biodegraded FMW as liquid fertilizer has been found to be a valuable resource for agriculture in our recent study [18, 19]. This is because FMW contains compounds that are capable of promoting plant growth [20]. Moreover, seafood-processing wastewaters do not contain known toxic or carcinogenic materials unlike other types of municipal and industrial effluents [16]. In our previous study [18, 19], the phytotoxicity of the biodegraded FMW was evaluated by using the seed germination test. Its result indicated the feasible development of a liquid fertilizer from FMW. More appropriate test for the fertilizing ability of liquid fertilizer is hydroponic culture as it provides a convenient means of studying plant uptake of nutrients that are free of confounding and uncontrollable changes in soil nutrient supply to the
roots [21]. Nowadays, hydroponics is considered as a promising technique not only for plant physiology experiments but also for commercial production [22, 23]. Therefore, an application of this culture technique can be considered as an alternate approach for large-scale production of some desired and valuable crops.
For the feasibility of commercialization, large-scale production of liquid fertilizer from FMW is necessary, as larger installations have advantages that result from the economy of scale [24]. However, transferring data that was obtained in lab-scale equipment to industrial production is not simple. This is due to the transport limitation which is considered as one of the major factors that is responsible for phenomena observed at large-scale [25]. Biological properties, especially various constants that are involved in kinetic equations depend on scale-up, although the metabolic patterns remain unchanged. Accordingly, it is necessary to investigate both the biological and technological aspects of the system in the large scale. For the commercialization of the liquid fertilizer, maintenance of its quality is also important during preservation. In our previous study [26], liquid fertilizer was produced from fish waste and its quality can be maintained by the addition of 1% lactate for 6 mon without any putrefaction. This was due to a bacteriostatic effect of lactate [27]. In this study, a large-scale bioconversion of FMW into liquid fertilizer was carried out in a 1 m3 reactor and the quality of the fermentation product as liquid fertilizer was analyzed for commercialization.
2.1. Microorganism and Its Maintenance
Mixture of microorganisms used in this study are comprised of seven microorganisms:
The FMW was obtained from a local fishmeal manufacturing factory. This was characterized as follows (per liter): 115,000 ± 13,000 mg of chemical oxygen demand (CODCr); 68,900 ± 7,600 mg of 5-day biological oxygen demand (BOD5); 15,400 ± 1,300 mg of total nitrogen (TN); 2,800 ± 600 mg of NH4 +-N; 0 mg of NO3 --N; and 0 mg of NO2 --N [18]. The initial pH of the FMW was 6.8 ± 0.2. The FMW was boiled to 80℃ for 3 hr in order to kill internal microorganisms and then, it was pumped into the reactor.
2.3. Production of Liquid Fertilizer
For commercialization, a scaled-up process was carried out in a 1 m3 reactor (Fig. 1). The reactor was preliminarily washed with 600 L of tap water that contains 5% of NaOH under vigorous air flowing to remove microorganisms attached on the surface of the reactor wall. Then, the reactor was sterilized by hot steaming at 3 kg/cm2 for 30 min and it was filled with 600 L of hot FMW under steaming. When the reactor temperature cooled down to 45℃, 110 g of bacteria paste (wet weight basis) that contains equal amounts from each seven strains were seeded to the reactor. In order to achieve faster biodegradation, the seeded cells were acclimated for 1 day in sterile FMW under an aerobic condition. The reactor was operated at 44 ± 3℃ by a cooling system (25 L/min of circulation). In the first experimental trial, air was supplied from a blower (capacity of 3.2 m3/min) through a ceramic disk-typed diffuser at an aeration rate of 320 L/min. As this amount of air was insufficient, air supply was gradually increased in later experimental trials by using two blowers (6.4 m3/min) and four diffusers (1,280 L/min). For better aeration, an oxygen generator (with 90 ± 3% purity) was used, instead of air blower. Oxygen flowed into the reactor at 5 L/min through a 0.01 μm filter and the size of the oxygen bubbles ranged from 0.1-0.6 mm. In order to avoid contamination from outer foreign microorganisms, discharging air or oxygen was passed through a 10-L plastic bottle that contained10-N NaOH. When severe foaming occurred during bioconversion, ten-fold diluted ‘Antifoam 204’ was used. Samples were periodically taken from the bioreactor. The concentrations of dissolved oxygen (DO), CODCr and TN were measured along with the oxidation-reduction potential (ORP) and pH.
The concentrations of CODCr and TN were analyzed by a Water- quality Analyzer (HS 2000; Humas Co. Ltd, Daejeon, Korea). The analysis of amino acids composition, seed germination test and hydroponic culture were performed for biodegraded FMW as a liquid fertilizer in order to examine its quality. The composition of amino acids in the biodegraded FMW was analyzed at Feeds and Foods Nutrition Research Center. According to the method of Wong et al. [28], the seed germination test based on the germination index (GI) value was carried out by using cress (
In this section, experimental results achieved in the scaledup process were analyzed for commercialization. The large-scale bioconversion of FMW into liquid fertilizer and the quality of the liquid fertilizer were compared with those achieved in lab-scales.
3.1. Production of Liquid Fertilizer
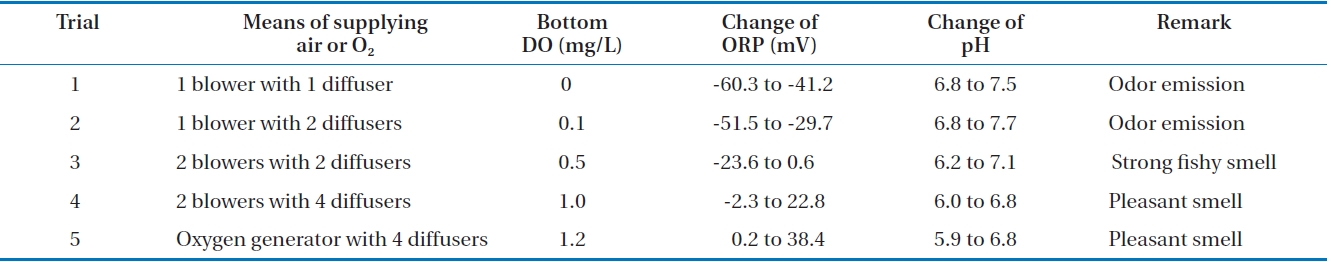
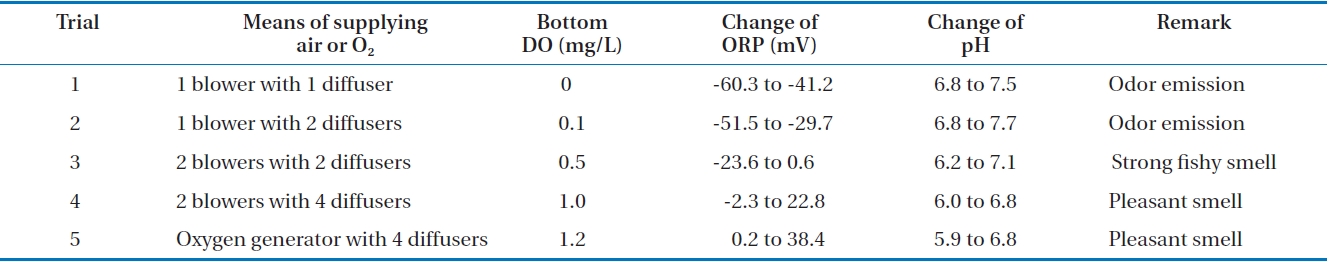
Experiments for the production of liquid fertilizer from FMW were tried five times in a 1 m3 reactor. The results are tabulated in Table 1. Even though the seven FMW-degrading microorgan-
[Table 1.] Comparison of the reaction characteristics among five trials executed in a 1 m3 reactor
Comparison of the reaction characteristics among five trials executed in a 1 m3 reactor
isms dominated during biodegradation, some contaminants were found at the end of the first trial. This was probably due to the lack of operation skill in large-scale. The operation skill in addition to sterilization of reactor in large-scale is important for commercialization, as it often influences the quality of the liquid fertilizer. However, the contamination problem was overcome. As seen in Table 1, values of the reaction parameters were dependent upon the means of supplying air or O2 to the reactor. When only one blower was used as an air supplier, DO level decreased to almost zero as the reaction occurred actively, ORP exhibited negative values (from -60.3 to -41.2 mV) and pH rather increased. This was due to the mixed microorganisms which were not able to adequately degrade the organic matter in FMW under insufficient DO and this results in odor emission [29]. This reaction was to some extent improved, when the two blowers were used with two diffusers. Under this situation, DO levels can maintain over 0.5 mg/L even at an active reaction and pH dropped to 6.2. Still, most of the ORP values (from -23.6 to 0.6 mV) were negative and strong fishy smell was emitted. It has been reported that DO level should remain higher than 1 mg/L during aerobic fermentation [30]. In this study, this criterion can be fit when two blowers with four diffusers were used. With this aeration setup, DO levels maintained over 1 mg/L all the time, ORP exhibited almost positive values (from -2.3 to 22.8 mV) and pH dropped to 6.0 under an active cell activity. Particularly, fishy smell was not emitted anymore and the same phenomenon was found in our previous study [19]. The effect of DO level on reaction parameters was rather observed more, when pure oxygen was supplied to the reactor through the four diffusers. Under this situation, DO levels maintained over 1.2 mg/L all the time, ORP exhibited all positive values (from 0.2 to 38.4 mV) and pH dropped to 5.9. From all the above results, it has been found out that aeration was a critical factor in a scaled-up process. Moreover, there was a clear relationship among the reaction parameters, DO and ORP.
In order to examine the change in the reaction parameters during the bioconversion of FMW into the liquid fertilizer, the best result achieved in the fifth trial is shown in Fig. 2. The DO level dropped over 6 hr as the mixed microorganisms started to degrade the organic matter in FMW. It dropped steadily to a bottom DO of 1.2 mg/L at 45 hr, and then it slightly increased. It has been known that oxygen is a key substrate in aerobic biodegradation and the continuous transfer of oxygen from the gas phase to liquid phase is critical for the maintenance of oxidative metabolism in the cells [19]. According to the report proposed by Tohyama et al. [30], the means of oxygen supply in this study revealed to be adequate in order to meet the minimum demand of oxygen for large-scale bioconversion. The pH was 6.8 at the beginning of biodegradation and it decreased to 5.9 after 47.5
hr due to the production of amino acids [29]. Then, the pH increased slowly to a final value of 6.04. The value of ORP started at 18.2 mV and increased to 38.4 mV after 20.5 hr. This was followed by a steady decrease to a bottom ORP value of 0.2 mV at 49 hr. In our previous study [19], the decrease in ORP value was found to be related to the decrease in DO. As a result, active bioconversion appeared to take place from 20.5 hr to 49 hr. Besides, maintaining positive ORP values resulted in the disappearance of a strong unpleasant fishy smell at the end of the bioconversion. It has been reported that ORP can be a good parameter for the oxygen supply [31]. Moreover, an unpleasant odor can be easily produced under incomplete aerobic biodegradation [32]. Therefore, the maintenance of positive ORP values during large-scale bioconversion was very important, as ORP was closely related to the DO level. As a result, process optimization is required in large-scale bioconversion, especially for aeration in this case. Nowadays, online ORP monitoring has been proved to be a practical and a useful technique for the process control of wastewater treatment systems [33, 34].
After 52 hr of bioconversion, the concentrations of CODCr and
Comparison of the characteristics of biodegradation among liquid fertilizers produced under different culture conditions
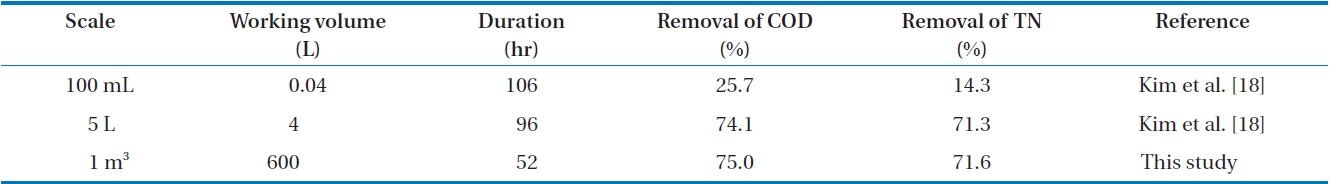
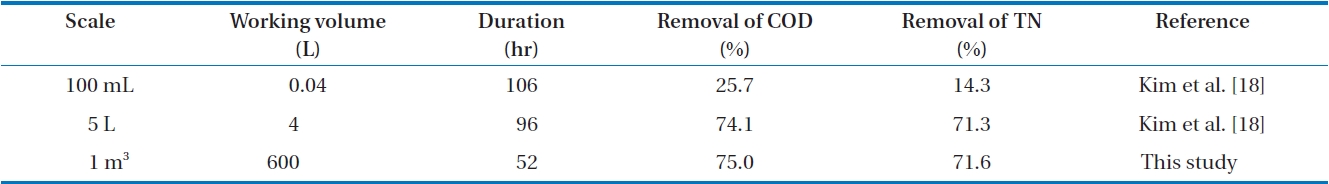
TN in FMW decreased to 28,700 and 4,380 mg/L, respectively. The average removal percentages of CODCr and TN were 75.0 and 71.6%, respectively. As described in our previous study [18] using the same mixed microorganisms, CODCr and TN were reduced while the mixed cells also grew with the production of CO2 and N2. This result was compared with those that were achieved in lab-scale [18]. The comparison is tabulated in Table 2. The degradation of the organic matter in FMW performed in a 1 m3 reactor took place at a much higher rate than that (25.7% of COD removal and 14.3% of TN removal) done in 100-mL scale. This indicates the importance of aeration with adequate mixing. Compared to that performed in a 5-L reactor, a scaled-up bioconversion was achieved in 44-hr shorter time with almost the same removal percentages of CODCr and TN. This implies that the transfer of lab-scale data to a large-scale production will be successful without transport limitation. It is known that the COD/TN ratio may influence the metabolic pathway of the organic matter utilization [35]. Accordingly, control of the COD/TN ratio may be necessary for better bioconversion. In this study, the ratio of CODCr/ TN decreased slightly from 7.5 to 6.6. This small change in the CODCr/TN ratio implies a relatively stable metabolism of the organic matter degradation.
3.2. Quality of Liquid Fertilizer
For commercialization, the fertilizing ability of the biodegraded FMW is important with non-toxicity. In our previous study [18], the feasibility of the biodegraded FMW was demonstrated in a lab-scale as liquid fertilizer for plant growth. It has been known that the biological properties such as metabolic reactions of microorganisms are dependent upon scale-up [25]. In comparison to the lab-scale, the typical differences in large-scale are reduced biomass yield, increased by-products production and the presence of limiting substrate gradient as measured at different heights in the bioreactor [36, 37]. Therefore, such quality of liquid fertilizer produced in large-scale was necessary to compare with those that were produced under different culture conditions. The result is tabulated in Table 3. In this study, GI test was taken at 1,000-fold dilution as 1,000-fold diluted liquid fertilizer was used in horticulture for general purpose [29]. The GI value was found to be 90% and this indicates phytotoxic-free fertilizer [38]. This GI value was comparable to that (92%) produced in a lab-scale. It was higher than that (80%) made from fish waste in lab-scale. It has been reported that phytotoxicity is caused by organic compounds such as ammonia and low molecular weight organic acids [39] and it can be remedied either by increasing the period of aerobic decomposition [28] or by reducing the concentration of the substrate [40]. As a result, liquid fertilizer produced in a 1 m3 reactor was found to be phytotoxic -free by adequate biodegradation.
It is known that many plants have transporters for the uptake
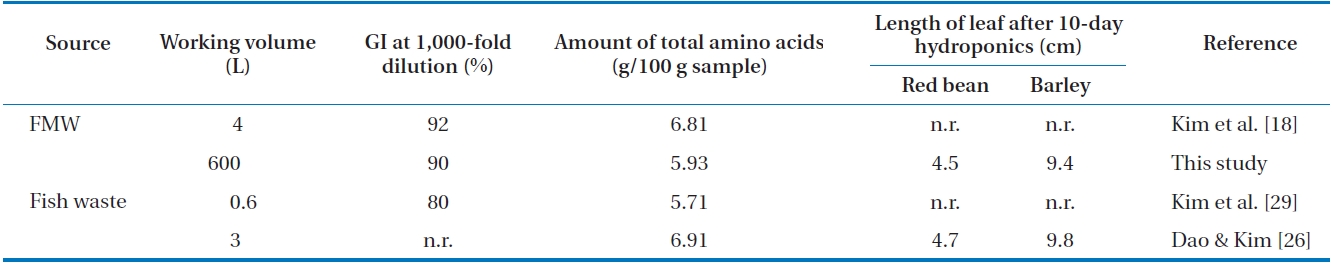
Comparison of quality among liquid fertilizers produced under different culture conditions
of organic forms of nitrogen, particularly amino acids [41, 42]. Moreover, amino acids can be an important source of plant N especially in a nutrient-limited natural ecosystem [43]. Based on this point of view, the amino acid composition of the 52-hr culture of inoculated FMW was analyzed, and the amount of total amino acids was compared to those produced under different culture conditions (Table 3). The amount of total amino acids produced in this study was 5.93 g/ 100 g sample. The composition of amino acids was similar to that produced in the lab-scale (data not shown). Amino acids are an essential part of the active fraction of organic matter in a fertilizer. Their composition will be used as a means of assessing bioconversion, as the growth of plants depends ultimately upon the availability of a suitable balance of amino acids. As a result, the quality of liquid fertilizer produced in a 1 m3 reactor was maintained in spite of scale-up. However, the level of amino acids was to some extent lower than that (6.81 g/ 100 g sample) produced in lab-scale. This difference may come from the different compositions of raw materials (fish waste) that were used for fishmeal manufacture. A portion of amino acids may also be utilized by the mixed microorganisms during the bioconversion, as amino acids have been reported to be used to improve protein yield [44]. Therefore, an appropriate control of large-scale process is necessary to maintain the quality of the liquid fertilizer.
In hydroponic culture, the length of a leaf was found out to be a prominent indicator for growth of red bean or barley [26]. According to this result, hydroponic culture was performed on a red bean seed or barley seed by using 52-hr culture of inoculated FMW. As seen in Table 3, the growth of either a red bean seed or barley seed on the 52-hr culture of inoculated FMW was comparable to that on liquid fertilizer made from fish waste in a lab-scale. This indicates that the fertilizing ability of the biodegraded FMW produced in a 1 m3 reactor was adequately good. From all the above results, the production of biodegraded FMW as liquid fertilizer was successfully carried out in a scaled-up process. Thus, the commercialization of liquid fertilizer made from FMW was feasible. Further process for commercialization, is a field experiment for the application of liquid fertilizer that was produced in large-scale to garden plants and it will be proceeding after this work.
FMW has been customarily disposed by dumping into the sea. However, there is an urgent need to find an ecologically acceptable means for the reutilization of FMW as dumping will be prohibited in Korea after 2012. Biodegraded FMW as a liquid fertilizer has been found to be a valuable resource for agriculture. For the feasibility of commercialization, a scaled-up bioconversion of FMW into a liquid fertilizer was demonstrated in a 1 m3 reactor. In terms of fertilizing ability, compared to the liquid fertilizer produced in lab-scale, the scaled-up production of liquid fertilizer was successful. Optimization of the DO level was found to be crucial under adequate aeration in order to maintain the quality of a liquid fertilizer in scaled-up process. Moreover, the commercialization of a liquid fertilizer made from FMW was feasible. These results can provide an answer to the impending domestic problem that concerns with the prohibition of dumping FMW into the sea.