Heavy metals contaminations are produced by human activities such as industrial and agricultural activities and the heavy metals in soil are not degraded by the natural ecosystem; therefore, hazardous matters accumulate in the soil [1, 2]. They are transported to plants or groundwater, and are toxic to the biological systems of human and animal environments via the food chain [3]. In certain cases, the heavy metals should be remediated using an appropriate method.
Remedial technologies for heavy metals-contaminated soils include containment methods such as physical, encapsulation, and vitrification;
EDTA, a representative chelating agent, can extract heavy metals from contaminated soils with high efficiency [8]. However, with EDTA it is difficult to treat the effluent solution after treatment due to low biodegradability, biological toxicity, and high cost for treatment [7, 9, 10]. Non humified organic acid, and weak organic acid such as citric, tartaric, oxalic, formic and fumaric acids are natural products of root exudates and plant and animal residue decomposition that can be used as a washing solvent with biodegradable characteristics [11-14]. Strong inorganic acid can be used for useful washing solutions in terms of reasonable cost and simple handling of the effluent solution. Even though the strong acid causes soil acidification, it is an effective solvant due to high its removal effciency on heavy metal extraction, especially hydrochloric acid [10].
The effectiveness of soil washing is dependent on process parameters: the extracting mode such as batch or column, solvent type and concentration, extracting time, solid to liquid (S/L) ratio, and soil physicochemical characteristics such as pH, organic content, particle size distributions, as well as contaminated metal type and its concentration [7].
In this study, characteristics of Pb desorption for remediation of the Pb-contaminated soil were evaluated using hydrochloric acid (HCl) by a washing method. Batch experiments were performed to identify the factors influencing extractive decontamination such as the S/L ratio, extracting time, particle size distributions and multi-stage washing. In addition, the Pb desorption was characterized by applying it to a kinetics model.
2.1. Soil Sampling and Characterization
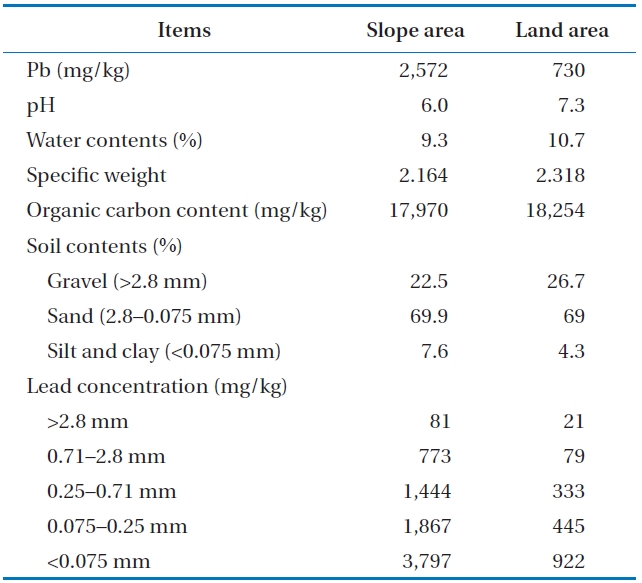
Soil samples were collected from two different areas: a slope area (SA) and a land area (LA) at a clay shooting range in Korea, which was highly contaminated with lead (Pb). The soil was sampled at a depth below 20 cm from the surface of each area. The collected soil samples were air-dried and analyzed for the following characteristics: initial pH, water content, specific weight, organic carbon content and Pb concentration for each sample. The lead bullet was removed from the initial soil sample for the accurate measurement of the contamination concentration of lead in the soil. Pb was pre-treated by the EPA Method 3050B [15] and analyzed using an atomic adsorption spectrometer (AA-6300; Shimadzu, Tokyo, Japan). All other tests followed the Korean standard method for soil pollution [16]. Particle size distribution was performed using the wet method by spraying with water (about 150 mL/900 g of soil). Soil was separated using 5 steps with the following particle sizes: >2.8, 2.8?0.71, 0.71?0.25, 0.25?0.075, <0.075 mm. Lead particles larger than 1 mm were manually removed for the entire examination.
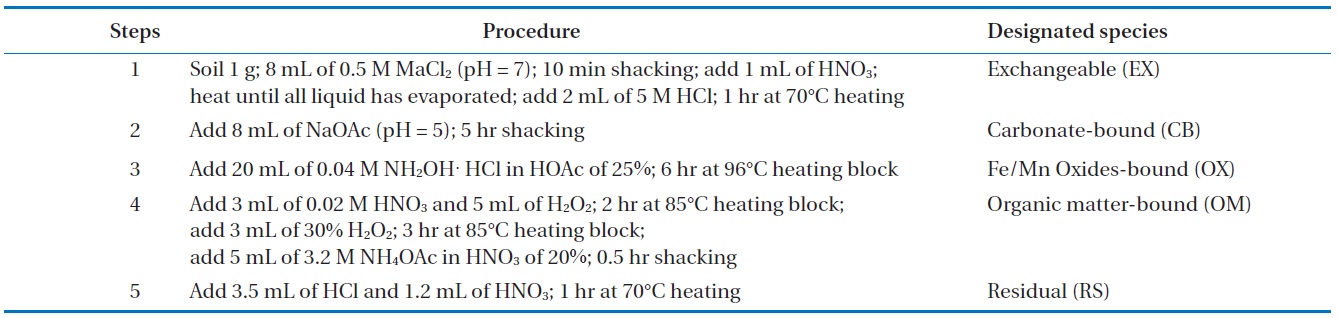
2.2. Sequential Extraction of the Soil
Soil samples with particle sizes of 2.8?0.075 were analyzed by the sequential extraction method [17] to identify the metal combined property in the soil. Heavy metals in the soil were sequentially extracted in different forms including exchangeable (EX), carbonate-bound (CB), Fe/Mn oxides-bound (OX), organic matter-bound (OM), and residual (RS). The designated portion of the soil was then examined using the sequential extraction method (Table 1).
2.3. Batch and Multistage Washing
Each soil sample separated according to particle size was examined for washing efficiency using HCl as a washing solution. Thirty grams of each soil sample was placed into three different Erlenmeyer flasks with a 0.1 M HCl solution with specific solid
[Table 1.] Sequential extraction procedure using 5 steps for heavy metals in soil
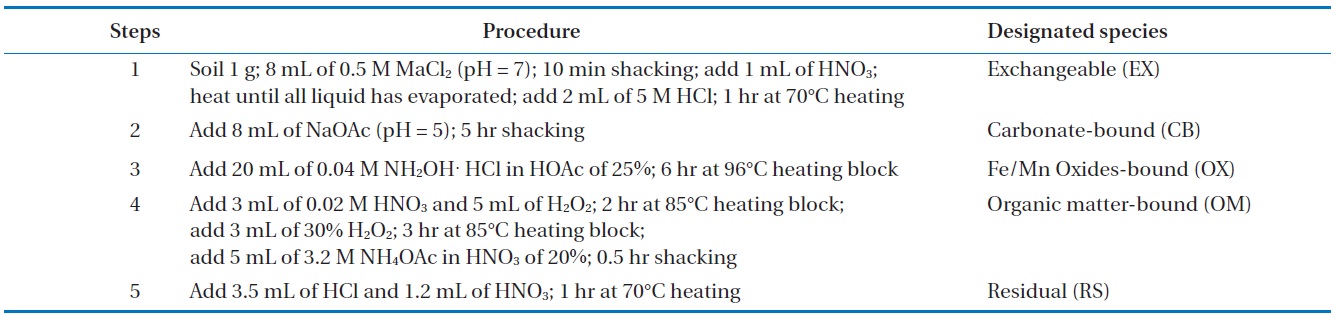
Sequential extraction procedure using 5 steps for heavy metals in soil
to liquid ratios of 1:2, 1:3, and 1:4 (g of soil to mL of HCl). The samples were mixed in a shaker at 300 rpm for 10 min. Multistage washing tests (3 stages) were sequentially examined with the most efficient mixing rate obtained from the above washing test at the same condition. The sample was settled down for a few hours until the soil and liquid phase became separated. After the phase separation, the liquid was replaced with a new washing solution. The lead concentration in the residual soil was analyzed at each stage. The added volume of the washing solution was adjusted according to the existing soil mass in the multistage washing test. The final efficiency and necessity of the multistage washing were determined throughout the test.
3.1. Physicochemical Properties of Soil
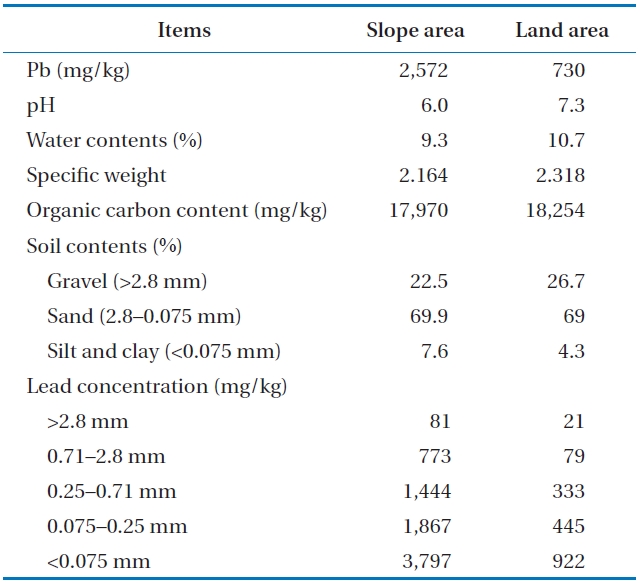
The properties of the soil examined are presented in Table 2. The initial lead concentrations contaminated at the slope and land areas were 2,575 mg/kg and 730 mg/kg, respectively. The pH ranged from 6 to 7.3 and the water contents were similar to those in the general range regarding sandy loam (10% to 18%). Organic carbon content was roughly 18%, indicating a strong potential for oxidization by acid to remove the heavy metals in the soil. The soil contents examined were separated in gravel (>2.8 mm), sand (2.8?0.075 mm), as well as silt and clay (<0.075 mm). Approximately 70% of the soil consisted of sand. The characteristics of the tested soil were adapted in order to use chemical washing as a method to remove the lead. The lead concentration in soil was permitted to be below 400 mg/kg by Soil Environmental Conservation Act (SECA) in Korea [18]. However, the lead concentration below a particle size of 2.8 mm exceeded the standard criteria in Korea. The lead concentration contaminated in the soil at the slope and land areas was significant in smaller sized particles. The soil with smaller sized particles provided more specific surface area that can combined with heavy metals. Therefore, the soil type and distribution characteristics are important process parameters.
The soil samples were extracted by the sequential method in order to identify the metal combined property. Extraction efficiency is not only affected by extraction parameters but also a combined form of heavy metals. In addition, the reaction rate between solution and metals, and movement characteristics in the soil are dependent on the combined metal property. Heavy metals are divided by non-detrital and detrital fractions. The non-detrital form of heavy metal can be easily removed by the washing method [19, 20]. In this study, 92.4% of the heavy metals in the soil at the SA existed in non-detrital forms (74.5% of EX, 17.2% of CB, and 0.7% of OM), and 7.7% of that in detrital forms (5.5% of OX and 2.2% of RS). Furthermore, 83% of the heavy metals in the soil at the LA existed in non-detrital forms (58.6% of EX, 23.4% of CB, and 1.0% of OM), and 17.1% of that in detrital forms (10.5% of OX and 6.6% of RS). Most heavy metals in soil generally existed in detrital forms, while most of the Pb in the examined soil existed in non-detrital forms, which can be easily removed by the soil washing method. Therefore, the analysis revealed that the washing method using solvent would be effective for remediation of Pb-contaminated soil in this study. In addition, the low rate of OM and RS forms of soil implies that the heavy metals were influenced from an external source, not from the soil mineral portion.
3.2. Washing Efficiency in Batch
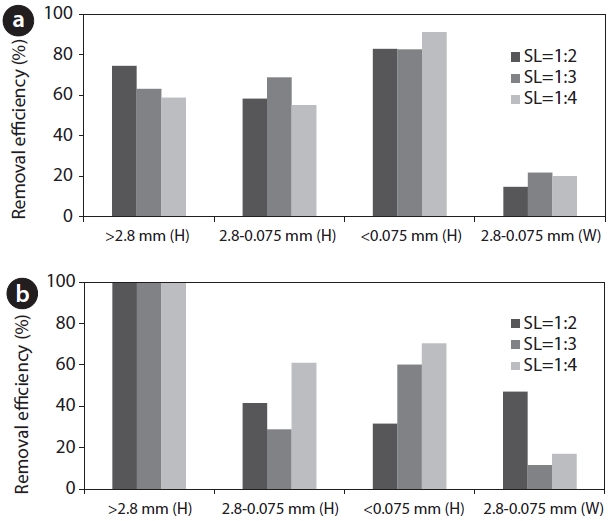
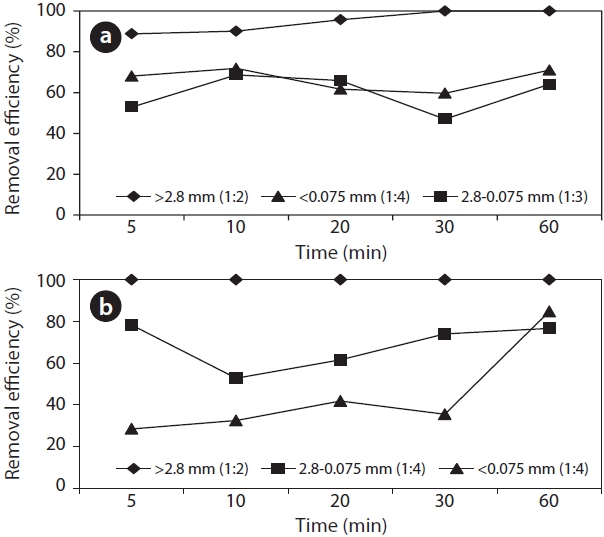
Pb-contaminated soil at the SA and LA was examined depending on the soil particle sizes and SL ratios. The Pb removal efficiency would be affected by the S/L ratio because when strong acid is used as a washing solvent, the form of Pb in the soil can be changed [21]. In addition, an optimal S/L ratio is required in terms of the treatment of wastewater produced after the washing event. Additionally, the removal efficiency would also be affected by the soil particle size because the surface area, where is possibly combinable with heavy metals, is high as particle size smaller. 0.1 M of HCl was used as a solvent and its efficiency was compared to that of water. The removal efficiency differed depending on the particle sizes and S/L ratios (Fig. 1). For the SA, with a soil particle size greater than 2.8 mm, the Pb removal efficiency was significant with a low S/L ratio (1:2). In contrast, the efficiency was significant with a high S/L ratio having a soil particle size below 0.075 mm. The optimal S/L ratios were 1:2 (>2.8 mm), 1:3 (2.8?0.075 mm), and 1:4 (<0.075 mm). At the LA, Pb was totally removed in the gravel sized soil (>2.8 mm) and the optimal S/L ratios were 1:4 in other particle sizes. When the HCl
Soil characteristics and lead concentration contaminated in the soil by the soil particle sizes
washing solution was used, the removal efficiency was greater than 54%, which was high compared to that of the water. Water also presented an average removal efficiency of 18?25% resulting from the low coherence between soil and Pb. This is because the soil was originally contaminated by lead bullets and in the soil, the lead exists in the particle phase by being crushing to a small size rather than by being strongly adsorbed in the soil.
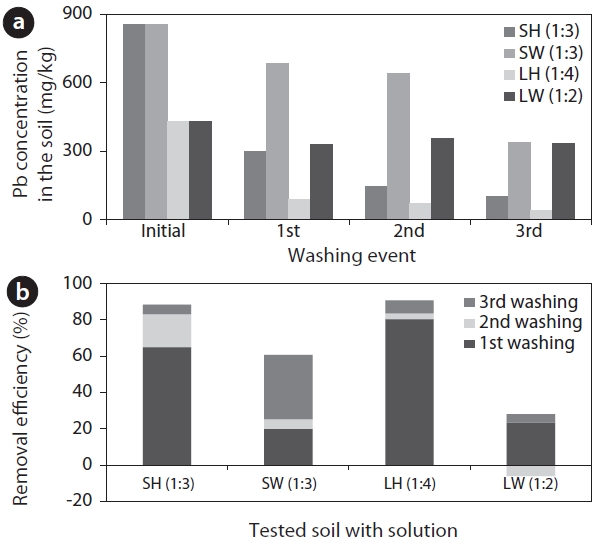
Sequential extraction via 3 steps was conducted with appropriate S/L ratios using 0.1 M of HCl and water. The experiments were achieved at a soil size of 2.8?0.075 mm because most of the Pb in the soil with particle sizes of below 2.8 mm and very small particle size soil (<0.075 mm) is difficult to handle in the field. In addition, the extraction efficiency was comparatively low in the batch experiment, requiring multi-stage treatment to meet standard criteria [16]. Therefore, the 2.8?0.075 mm of soil was examined at a different S/L ratio, which is the optimal ratio resulting from the batch experiment using HCl or distilled water (Fig. 2). When the soil was treated with HCl, approximately 65% to 80% of Pb was extracted at the first washing. The extraction efficiency decreased compared to the first washing event, but the final extraction efficiency reached around 90% at the following washing event. The highest extraction efficiency was present at the first washing event due to the low coherence between Pb and the soil while the extraction tendency was unclear with water as a washing solution.
3.4. Effect of Extraction Time
The optimal extraction time was determined depending on the soil particle size with the proper S/L ratio. The extraction time was set at 5, 10, 20, 30, and 60 min and the results are presented in Fig. 3. A particle size greater than 2.8 mm presented high removal efficiency in both testing soil samples resulting from the initially low contamination. The extraction time was insignificant to the efficiency for the soil with soil particles sized
2.8 to 0.075 mm, which is a practical target for treatment at the SA in Fig. 3(a). The extraction time was insignificant for soil particles smaller than 0.075 mm and removal efficiency averaged roughly 66%.
The optimal extraction time was 5 min for the soil (2.8?0.075 mm) from the LA while the removal efficiency with the soil (<0.075 mm) was slightly increased until 30 min and dramatically increased at 60 min. This is because the initially low Pb concentration (922 mg/L) could contribute to higher removal efficiency by increasing the extraction time compared to that from the SL soil (3,797 mg/L).
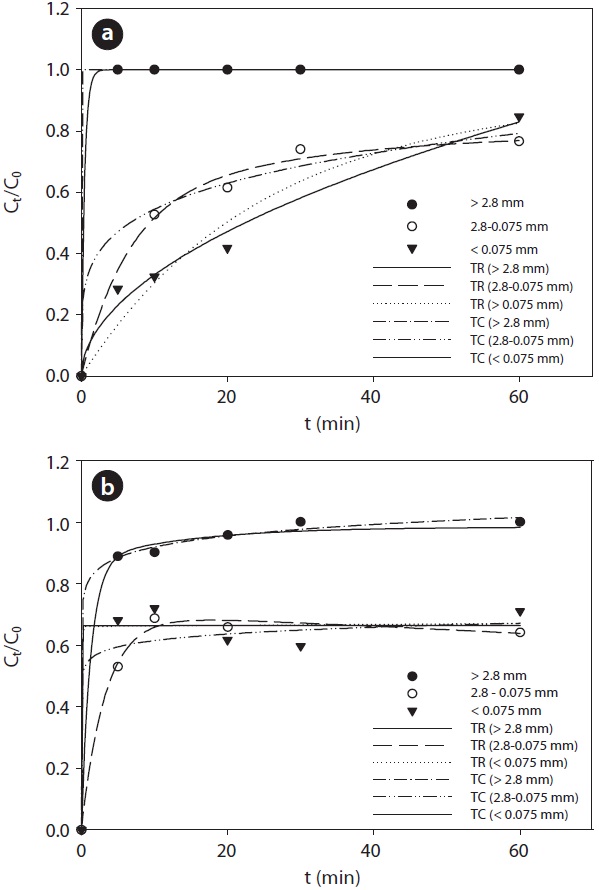
The desorption rate of Pb from the soil depending on the extraction time for different soil particle sizes was determined by the kinetics model. Desorption kinetic analysis was conducted using two reaction models and two constant kinetic models, which are general equations used to describe the characteristics of the desorption mechanism in soil [22, 23]. The two-reaction model (Eq. 1) and two-constant model (Eq. 2) equations are as follows:
where
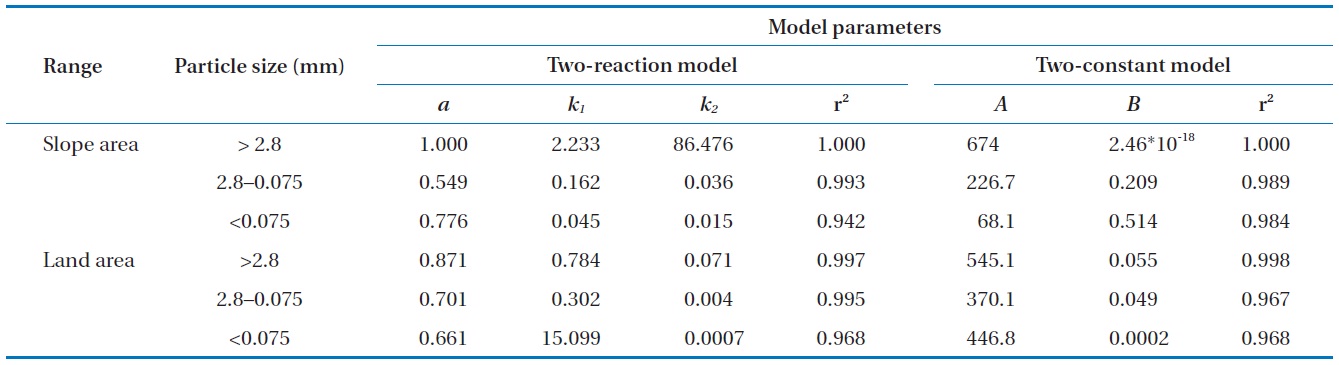
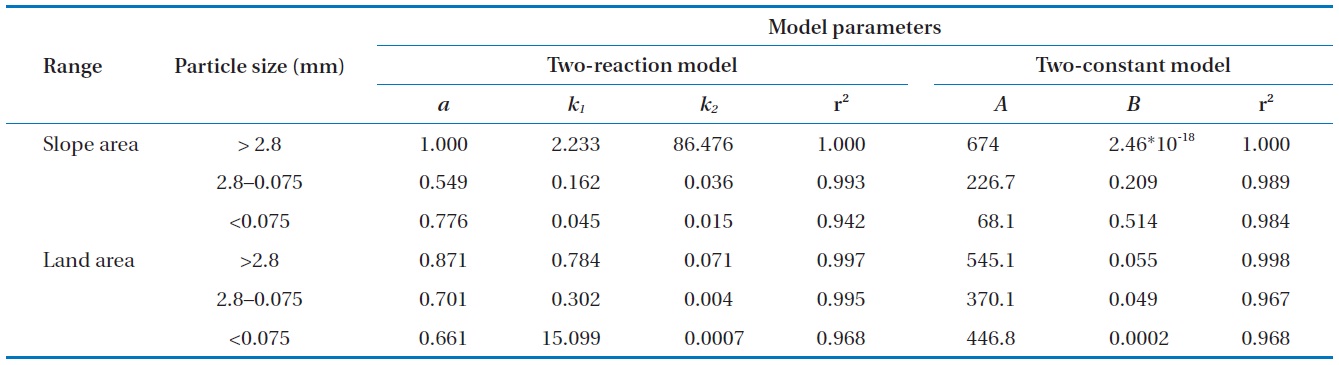
Both the two-reaction model and the two-constant model fitted well to the data in this study (Fig. 4). Especially, the Pb desorption characteristic was adequately described by the two-reaction model with a higher coefficient of determination. Hwang
[Table 3.] Parameters of kinetic models for lead extraction from soil
Parameters of kinetic models for lead extraction from soil
et al. [22] also presented similar results demonstrating that the desorption characteristic of Pb-contaminated soil was well described with the two-reaction model. The specific parameters of each kinetic model are presented in Table 3. Both soil samples with particle sizes greater than 2.8 mm were rapidly extracted within a short reaction time. The results were associated with the high ratio of extracted Pb at the initial rapid reaction step, which is
The Pb-contaminated soil at a clay shooting range was remediated by the soil washing method using 0.1 M HCl solvent caused by the weak coherence between the soil and Pb. The Pb in the soil mostly existed in an exchangeable form that could be easily released from the soil. The Pb was removed from the soil within 5 to 10 min with 65?80% of the removal efficiency occurring during the first washing event. The soil washing efficiency was significantly affected by the S/L ratio and soil particle distribution. The characteristic of desorption was well described by the two-reaction kinetic model. The acidification of the soil by the washing event using a strong acid would possibly influence the increase of movement of Pb in the soil. Therefore, continuous observation and management in regards to the contaminated soil would be necessary.