Since methicillin was introduced in 1959 to resolve infec-tions caused by penicillin-resistant
In an effort to decrease usage of vancomycin and discover an alternative therapeutic agent for treating MRSA infection, we have screened marine algae for anti-MRSA compounds.
>
Raw materials and extraction
In late September 2010, the brown seaweed
Standard bacterial strains were obtained from the Ko-rean Collection of Type Cultures (KCTC; Daejeon, Korea) and the Korean Culture Center of Microorganisms (KCCM; Seoul, Korea). The bacterial strains used were methicillin-susceptible
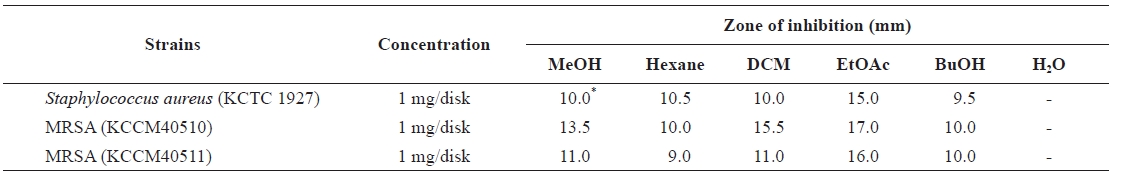
Antibacterial activity was evaluated by a disk diffusion assay, as described by the National Committee for Clinical Labo-ratory Standards (National Committee for Clinical Laboratory Standards, 2004). In brief, bacterial strains were cul-tured in TSB at 37℃ until an OD600 of 0.5. Bacterial culture (1 mL), containing approximately 104 CFU, was spread on a MHA plate, and a paper disk (6 mm diameter) containing 1 mg extract was then placed on the agar surface. After incuba-tion for 24 h at 37℃, the diameter of the inhibition zone was measured. The determination was performed three times and the mean values presented.
>
Minimum inhibitory concentrations (MIC)
The MIC is the lowest concentration of antimicrobials that will inhibit the visible growth of microorganisms after over-night incubation (Grierson and Afolayan, 1999). MICs of the extracts and vancomycin were determined by the twofold seri-al dilution method in MHB (NCCLS, 2003). MIC was defined as the lowest concentration of crude extract that inhibited vi-sual growth after incubation at 37℃ for 20-24 h, and was per-formed in triplicate (Grierson and Afolayan, 1999).
>
Minimum bactericidal concentrations (MBC)
The MBC value was defined as the lowest concentration of
>
High-performance liquid chromatography
To identify the active compound(s) in
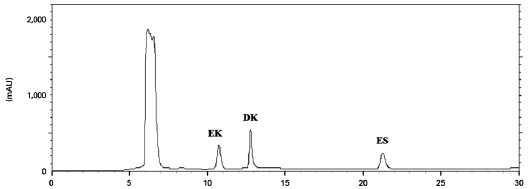
Standard phlorotannins (dieckol, eckol, and eckstolonol) were used to allow quantification. Standard compound con-centrations were calculated in the range of 0.01 to 0.1 mg by linear regression from the respective calibration curves. Eckol (Y = 12.973X - 0.4261; -
>
Anti-MRSA activity of E. bicyclis extract
>
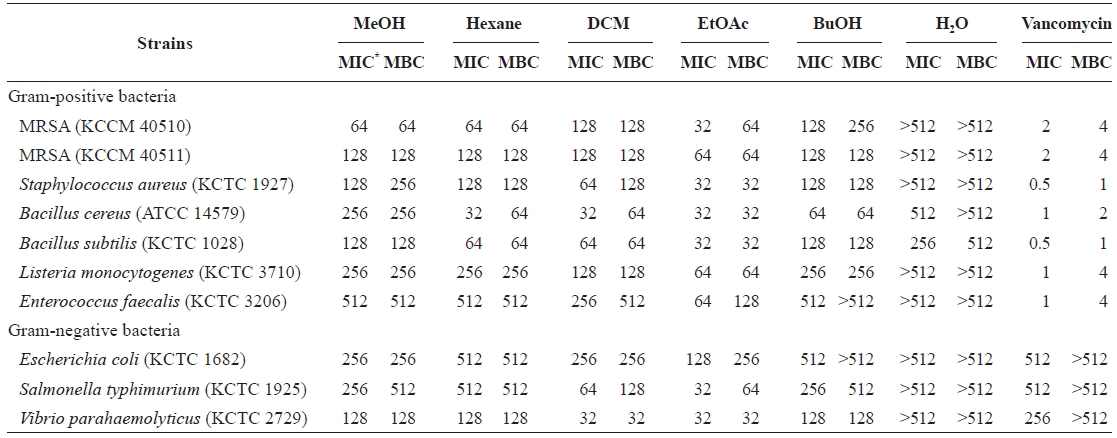
Determination of the MIC and MBC of E. bicyclis extract
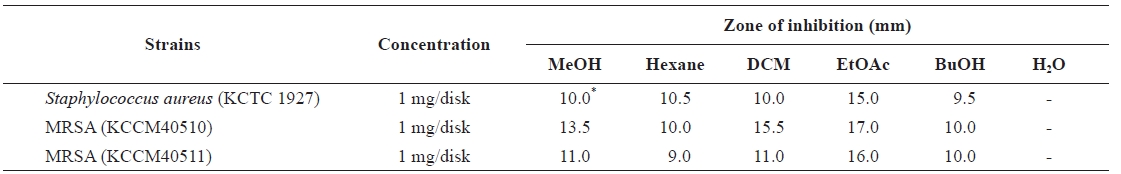
Growth inhibitory of Eisenia bicyclis extracts against methicillin-resistant Staphylococcus aureus (MRSA) and other strains
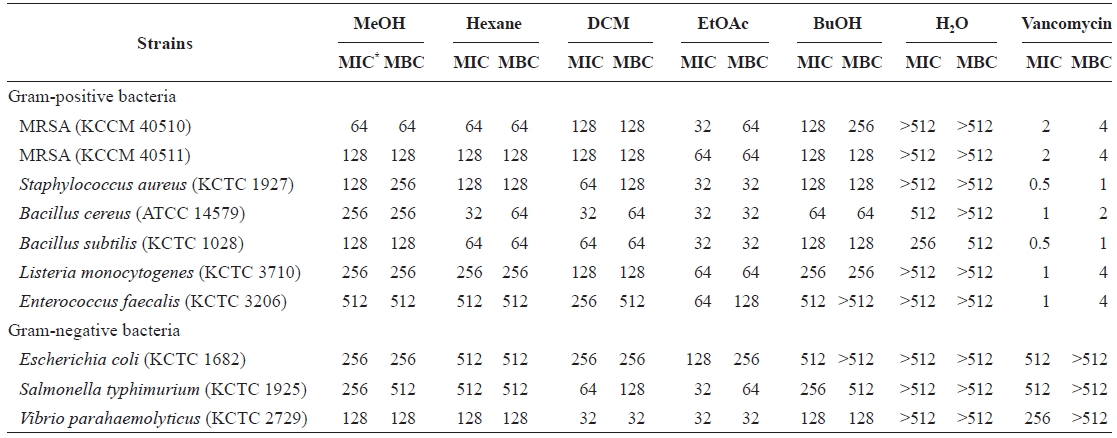
Minimum inhibitory concentrations (MICs) and minimum bactericidal concentrations (MBCs) of Eisenia bicyclis extracts against methicillin-resistant Staphylococcus aureus (MRSA) and other strains
The current study focused on the antibacterial activity of
Kim et al. (2002) reported that the EtOAc extract of this seaweed showed the highest antibacterial activity against the cavity-causing Gram-positive bacterium
Vancomycin, a tricyclic glycopeptide antibiotic, is used to treat MRSA infections (Lee et al., 2008). Vancomycin in-terferes with bacterial cell wall synthesis, as does penicillin, eventually leading to cell lysis (Barna and Williams, 1984). Most Gram-negative bacteria are less sensitive to vancomycin than Gram-positives (Totsuka et al., 1999; Lee et al., 2008). As expected, the MICs of vancomycin against Gram-neg-ative bacteria were over 512 μg/mL compared to 0.5-4 μg/mL against Gram-positives (Table 2). Unlike vancomycin, the EtOAc fraction exhibited strong antibacterial activity (MICs 32-128 μg/mL; MBCs 32-256 μg/mL) against Gram-negative bacteria. These data suggest the existence of different mecha-nisms of inhibiting cell growth and bacterial cell wall synthe-sis.
>
Identification of an anti-MRSA substance from E. bicyclis
The biological activities of plant materials are related to their phenolic compound content (Kim et al., 2006; Lin et al., 2008). Such activity is based on the physiological functions of polyphenol polymers (McDougall et al., 2005). Seaweed polyphenol (phlorotannin) is the predominant EtOAc-soluble compound in brown algae (Lee et al., 2008; Choi et al., 2010). Phloroglucinols, such as eckol, phlorofucofuroeckol-A, diek-
col, and 8,8′-bieckol, also exhibit antibacterial activity (Isnan-setyo et al., 2001; Nagayama et al., 2002).
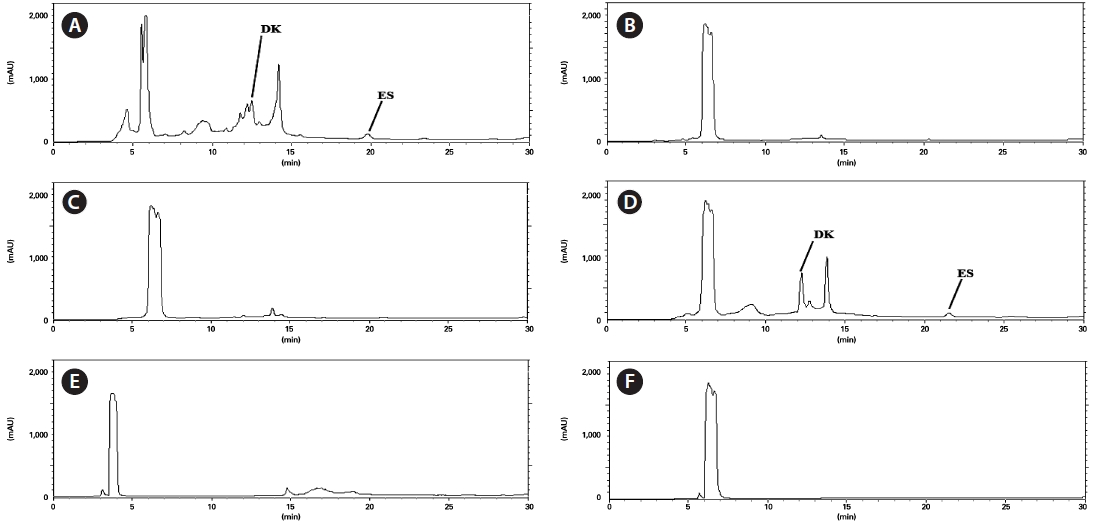
We detected anti-MRSA activity in
soluble fraction contained sizeable quantities of dieckol (76.9 mg/g in the EtOAc-soluble fraction) (Table 2).
Dieckol is a known antibacterial substance with activity against MRSA (Lee et al., 2008). Of the soluble fractions, di-eckol was present only in the EtOAc fraction, which exhibited the highest anti-MRSA activity. Our previous report showed that of the pholorotannins tested, dieckol showed the highest anti-MRSA activity (Lee et al., 2008). Considering these data, we suggest that the anti-MRSA activity of