The antioxidant and cholinesterase inhibitory activities of methanol, pretanol, and acetone extracts of Eupausia superba were in-vestigated and their bioactivities compared. The 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2'-azino-bis[3-ethylbenzthiazoline-6-sulfonic acid] (ABTS+) radical-scavenging activities and reducing power assays were used to determine antioxidant activities, and Ellman’s colorimetric methods were applied to evaluate cholinesterase inhibitory activity. Although all extracts were positive, Acetone extract of E. superba showed the highest activities. However, these showed moderate or no inhibitory activity against butyrylcholinesterase. Moreover, the total carotenoid contents of the organic solvent extracts followed the same order as their antioxidant and acetylcholinesterase inhibitory activities. These results suggest that E. superba is a potential source of natural antioxidants and cholinesterase inhibitors.
Alzheimer’s disease (AD), the most common type of senior dementia, is characterized by the progressive degeneration of neurological function (Nie et al., 2009). The pathogenesis of AD is associated with a reduction in cholinergic neurotrans-mitter levels in the basal forebrain, resulting in memory loss and reduced cognitive ability (Felder et al., 2000). AD can be prevented by cholinergic agents that recover the cholin-ergic functions through the inhibition of acetylcholinesterase (AChE) and butyryl-cholinesterase (BChE), which hydrolyze neurotransmitters such as acetylcholine (ACh) and butyrylcholine (BCh) (Schneider, 2001).
Oxidative stress caused by free radicals and reactive oxy-gen species (ROS) contributes to oxidation of biomolecules and cellular damage (Zhu et al., 2004). Recently, oxidative stress was related to the pathological changes in AD (Prat-ico and Delanty, 2000). Interest in the discovery of natural antioxidants from marine sources is growing because such compounds prevent oxidative damage and neurodegenerative diseases (Fusco et al., 2007).
The Antarctic krill,
In this study, the antioxidant and cholinesterase (ChEs) in-hibitory activities of
Whole
>
DPPH radical-scavenging activity
The DPPH radical-scavenging activity was measured by modifying the method of Blois (1958). An aliquot (160 μL) of sample in MeOH was added to 40 μL of 0.15 mM DPPH solu-tion. After mixing and leaving for 30 min at room temperature, the absorbance at 520 nm was measured using a spectropho-tometer (Powerwave XS; BioTex, Inc., Houston, TX, USA). The DPPH radical-scavenging activity of each sample was ex-pressed as an IC50 value, indicating the concentration required for scavenging 50% of the absorbance of the DPPH radical. ?-Ascorbic acid was used as a positive control.
>
ABTS+ radical-scavenging activity
ABTS+ radical-scavenging activity was determined by modifying the method of Arnao et al. (2001). The stock solu-tions were 7.4 mM ABTS+ and 2.6 mM potassium persulfate. The working solution was prepared by mixing the two stock solutions in equal quantities. The mixture was allowed to react for 12 h at room temperature in the dark, followed by dilution by mixing 1 mL ABTS+ solution with 50 mL MeOH to obtain an absorbance at 734 nm of 1.10 ± 0.02, as determined using a spectrophotometer (BioMate 5; Thermo Electron, Waltham, MA, USA). Fresh ABTS+ solution was prepared for each as-say. Sample (150 μL) was mixed with 2.85 mL ABTS+ solution and the mixture was left in the dark for 2 h. The absorbance at 734 nm was then measured using a spectrophotometer. A standard curve of trolox ranging from 9.4 to 37.5 μg/mL was prepared and the results were expressed as trolox equivalents per gram of extract.
Reducing power was evaluated by the method of Oyaizu (1986). Various sample concentrations (2.5 mL) were mixed with 2.5 mL of 200 mM sodium phosphate buffer (pH 6.6) and 2.5 mL of 1% potassium ferricyanide. After incubation at 50℃ for 20 min, 2.5 mL of 10% trichloroacetic acid (w/v) was added. The mixture was then centrifuged at 2,000 g for 10 min, and 5 mL of the upper layer was mixed with deionized water and 1 mL of 0.1% ferric chloride. The absorbance at 700 nm was measured using a spectrophotometer (BioMate 5). ?-Ascorbic acid was used as a positive control.
>
ChEs inhibitory activity assay
ChEs inhibition was measured using the spectrophotomet-ric method of Ellman et al. (1961). The reaction mixture con-tained 140 μL of 100 mM sodium phosphate buffer (pH 8.0), 20 μL of sample, and 20 μL of either AChE (0.36 U/mL) or BChE (0.36 U/mL). The solution was placed in a 96-well mi-croplate and mixed. After incubation at room temperature for 15 min, 10 μL of the DTNB solution and 10 μL of ACh or BCh, respectively, were added. The absorbance of all reac-tions was measured using a spectrophotometer (Powerwave XS). Eserine was used as a positive control.
A spectrophotometric method was used to evaluate the total carotenoid contents following the modified method of Tolasa et al. (2005). Astaxanthin standard (3.0 mg) and BHT (100 mg) were dissolved in 10 mL of dichloromethane. Subse-quently, 1 mL of this stock solution was diluted to 10 mL with
Castaxanthin (μg/mL) = A × 10,000/E,
where Castaxanthin is the total carotenoid content, A is the absor-bance at 472 nm, E = 2100 is the extinction coefficient, and 10,000 is the scale factor.
To prepare the standard curve, 0.1, 0.25, 0.50, 0.75, 1.0, 1.25, and 1.5 mL of stock solution were placed in separate 10 mL flasks using a solvent dispensing pipette and made up to the appropriate volume with
Oxidative stress is associated with age-related neurodegen-erative diseases (Mount and Downton, 2006). ROS oxidize and damage nucleic acids, lipids, and proteins. These reac-tions contribute to brain aging and age-associated neurodegen-erative diseases such as AD, likely because of the imbalance between antioxidant defenses and intracellular generation of ROS. Antioxidants play a crucial role in reducing unsaturated fatty acid oxidation in the brain and in preventing the neuronal death associated with the pathology of neurodegenerative dis-orders (Ramassamy, 2006; Kamatou et al., 2008).
DPPH and ABTS+ radical-scavenging activities and reduc-ing power were used to determine
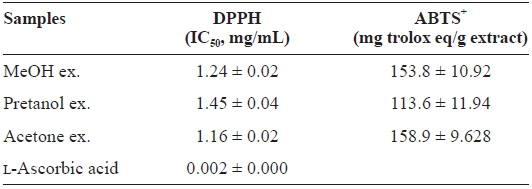
[Table 1.] DPPH and ABTS+ radical-scavenging activities of the extracts of Eupausia superba
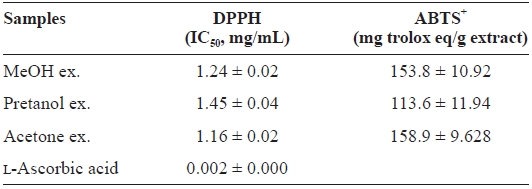
DPPH and ABTS+ radical-scavenging activities of the extracts of Eupausia superba
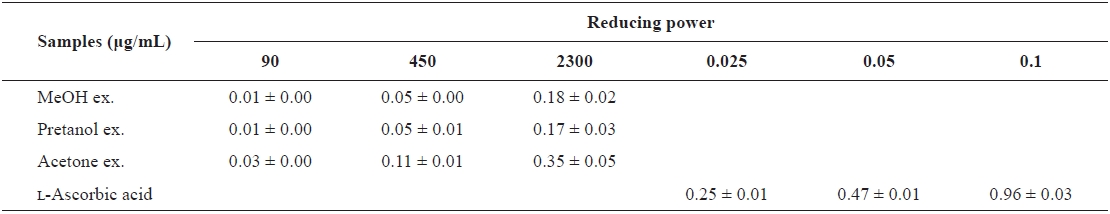
[Table 2.] The reducing power of the extracts of Eupausia superba
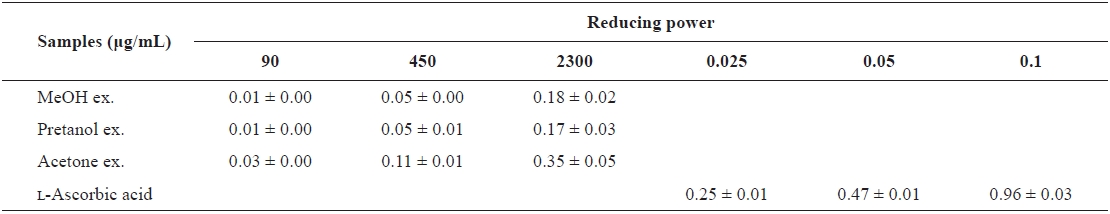
The reducing power of the extracts of Eupausia superba
extract) > pretanol ex. (113.6 ± 11.94 mg trolox eq/g extract). As summarized in Table 2, the reducing power of
AChE, a substrate-specific enzyme, exists in nerve syn-apses and catalyzes the cleavage of ACh in the synaptic cleft, which plays an important role in the initial stage of AD. BChE is a less-specific enzyme located in plasma and tissues, and lingers as the major ChE in the late-stage AD brain (Ballard et al., 2005; Silman and Sussman, 2005). Thus, inhibition of ChEs shows promise as an anti-AD therapy, and it has been shown to reverse the reduced cognition and behavioral func-tions associated with AD in clinical studies (Giacobini, 2004).
The ChEs inhibitory activity of
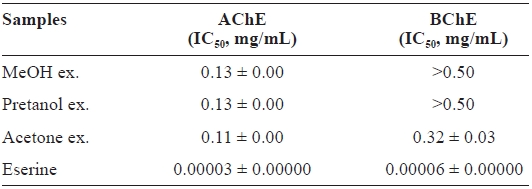
[Table 3.] Cholinesterase inhibitory activity of the extracts of Eupausia superba
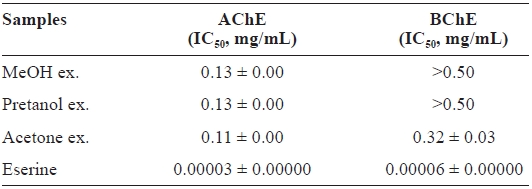
Cholinesterase inhibitory activity of the extracts of Eupausia superba
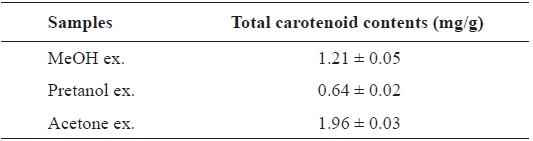
[Table 4.] Total carotenoid contents of the extracts of Eupausia superba
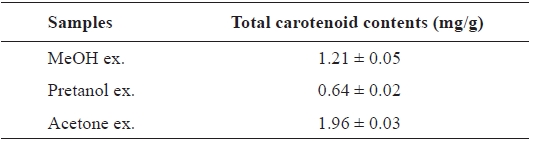
Total carotenoid contents of the extracts of Eupausia superba
and 0.32 ± 0.03 mg/mL, respectively, for AChE and BChE.
This selective AChE inhibitory activity may be due to the characteristics of enzyme-substrate binding (Silman and Suss-man, 2005). As with antioxidant activities, the ChEs inhibitory activity of
The carotenoids, a class of hydrocarbons with cyc-lic or acyclic end groups, exist as a pigment in crustaceans and exert biological effects such as antioxidant activity and prevention of cardiovascular disease and cancer (Britton, 1995; Kohlmei-er and Hastings, 1995; Stahl et al., 1998; Fraser and Bramley, 2004). The total carotenoid content of the
Thus, the order of total carotenoid content was similar to those of the antioxidant and ChEs inhibitory activities. Thus, these activities may be attributable to carotenoids. More de-tailed investigations are necessary to isolate and identify the active ingredients from extracts and to clarify their mecha-nism of action.