A 7-week feeding trial was conducted to investigate the effect of dietary starch level and kind on the growth and body composi-tion of juvenile olive flounder. Triplicate groups of fish (average weight: 1.5 g) were fed iso-nitrogenous (48% crude protein) and isocaloric (4.8 kcal/g diet) diets containing 15-25% α-potato starch and 15% β-potato starch. Survival was not affected by dietary starch level and kind. The weight gain of fish fed the diet containing 20% α-potato starch was significantly higher than that of fish fed the diets containing 15% and 25% α-potato starch levels. The feed efficiency and protein efficiency ratios of fish fed the diets containing 15% β-potato starch were significantly lower than those of the other groups (P < 0.05). The protein efficiency ratio tended to increase with increasing α-potato starch. The daily feed intake of fish fed the diet containing 15% β-potato starch was significantly higher than that of the other groups (P < 0.05). The hepatosomatic index, condition factor, and proximate composition of the whole body were not affected by the dietary starch level and kind. These results indicate that up to 20% α-potato starch could be incorporated into the juvenile flounder diet for optimum growth.
The ability of fish to utilize carbohydrates varies among fish (Wilson, 1994; Hutchins et al., 1998). The carbohydrate utilization of most carnivorous fish is generally lower than that of herbivorous or omnivorous fish. Although no precise carbohydrate requirements of fish have been reported, provid-ing an adequate level of carbohydrate in a fish diet may be very important to generate the least-cost formulation and to reduce catabolism of protein for energy. The maximum di-etary dextrin levels that did not reduce growth were 10% for yellowtail, 20% for red sea bream, and 30% for common carp (Millikin, 1982).
Flounder (
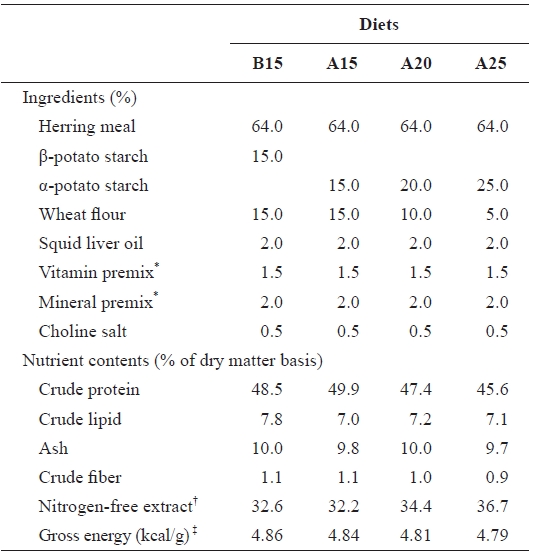
The ingredients and nutrient contents of the experimental diets are presented in Table 1. The four experimental diets were formulated to contain 15% β-potato starch (B15) and 15-25% α-potato starch (diets A15, A20, and A25, respectively) with iso-nitrogenous (48% crude protein) and isocaloric (4.8 kcal/g diet) diets according to the result of a previous study (Lee et al., 2002b). To determine the effects of various levels of starch, the amount of gelatinized starch in the diets was increased at the expense of wheat flour. Lipid levels were maintained at 7% (Lee et al., 2002a). For preparing the ex-perimental diets, calculated quantities of air-dried ingredients were thoroughly mixed in a Hobart electric mixer. The ex-perimental diets were formulated using a laboratory pellet ma-chine after 35-40 g water was mixed with a 100-g mixture of ingredients and dried at room temperature overnight. All diets were stored at -30℃ until use.
Juvenile flounder were obtained from a local farm (Uljin, Korea). The fish were acclimated to laboratory conditions for
[Table 1.] Ingredients and nutrients content of the experimental diets
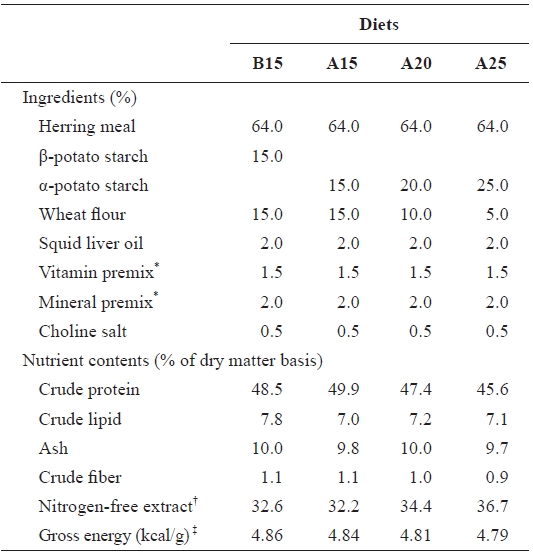
Ingredients and nutrients content of the experimental diets
2 weeks before the start of the feeding trial. Juvenile fish (ini-tial mean weight, 1.5 ± 0.04 g) were allocated randomly into twelve 260-L cylindrical plastic tanks (180 L water volume) with 35 fish per each tank for the feeding trial after being col-lectively weighed. Three replicate groups of fish were hand-fed to apparent satiation three times a day (08:00, 13:00, and 17:00 for 7 days per week) for 7 weeks. Filtrated seawater was supplied at a flow rate of 4 L/min in each tank and the mean water temperature and salinity were 20.6 ± 1.50℃ and 34 ± 0.1 ppt, respectively. The photoperiod was left under natural conditions during the feeding trail. Records were kept of daily feed consumption, mortalities, and feeding behavior.
>
Sample collections and analytical methods
At the end of the feeding trial, all of the fish in each tank were collectively weighed after anesthetizing with tricaine methanesulfonate (MS222; Sigma, St. Louis, MO, USA) at a concentration of 100 ppm after starvation for 24 h. Blood was drawn from the caudal vessel with 1 mL heparinized syringes from three fish in each tank and transferred to microcentrifuge tubes. The collected blood was centrifuged at 3,500 × g for 5 min and the plasma was separated and stored in a -75℃ freezer. Before freezing, plasma was divided into separate ali-quots for analyses of glucose, total protein, and total choles-terol in the plasma. The remaining fish from each tank were used for proximate analyses. The crude protein content was determined using the Kjeldahl method with the Auto Kjeldahl System (Buchi, Flawil, Switzerland), the crude lipid content was determined by the ether-extraction method, the moisture content was determined with a dry oven (105℃ for 24 h), and the ash content was determined by muffler furnace (550℃ for 6 h). Plasma glucose, total protein, and cholesterol levels were determined using a clinical investigation commercial kit (Asan Pharmaceutical Co., Seoul, Korea).
The data were subjected to one-way analysis of variance (ANOVA) using SPSS version 10.0 (SPSS Inc., Chicago, IL, USA). Significant differences (
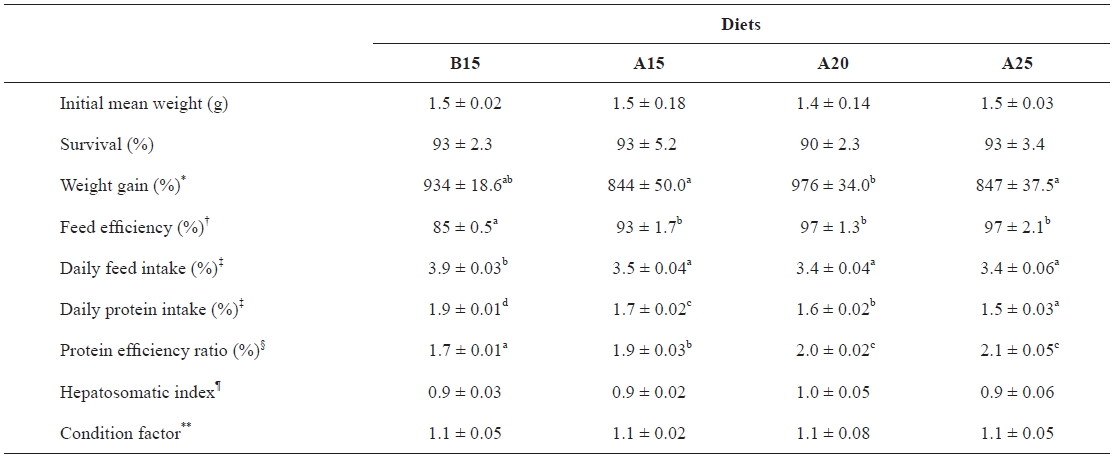
The growth performances and feed utilizations of juve-nile olive flounder fed the experimental diets for 7 weeks are shown in Table 2. The survival rates of the fish in each dietary group were 90-93% with no difference among treatments. Weight gain was affected by dietary α-starch level (
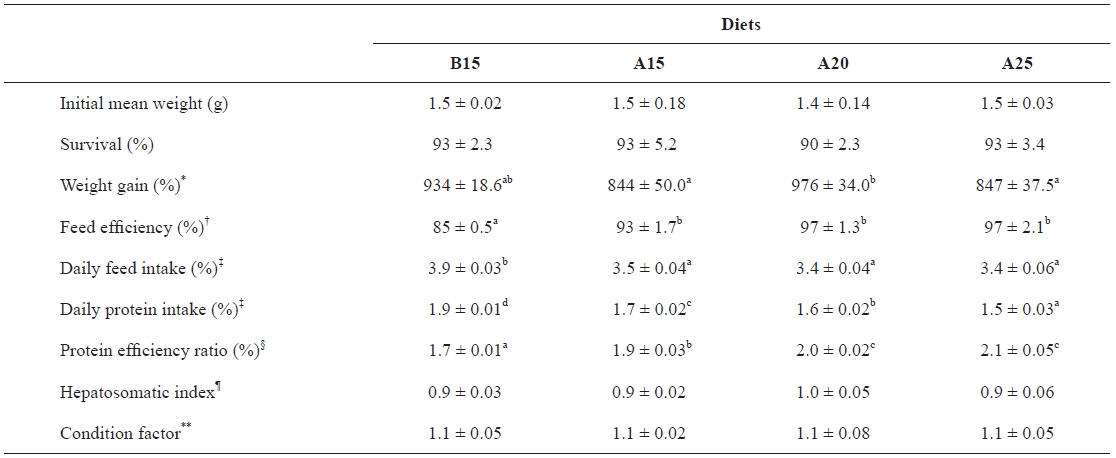
Growth performance of juvenile flounder fed diets containing different starch level for 7 weeks
diets containing 20% α-starch. Feed efficiency was affected by the dietary starch kind at the same level (
Minimizing feed costs for fish production in aquaculture can be achieved by determining the requirements of high-cost essential nutrients such as protein while maximizing the use of low-cost energy sources. Carbohydrates are the most cost-effective energy source for fish, although the abilities of fish to utilize carbohydrates vary among species. The use of dietary carbohydrates by fish appears to be related to their digestive systems and metabolic pathways adapted to a variety of aquat-ic environments (Walton and Cowey, 1982) as well as dietary carbohydrate level and complexity (Bergot, 1979; Hutchins et al., 1998; Small and Soares, 1999). Generally, herbivorous or omnivorous fish use much higher levels of carbohydrates than carnivorous coldwater fish such as marine fish and salm-on (Wilson, 1994). We found that the weight gain of olive
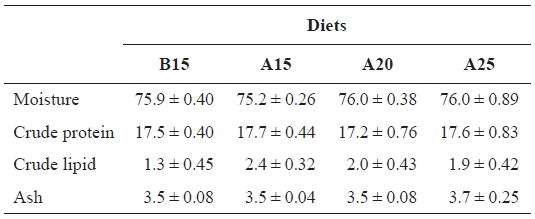
[Table 3.] Proximate composition (%) of whole body of juvenile flounder at the end of feeding trial
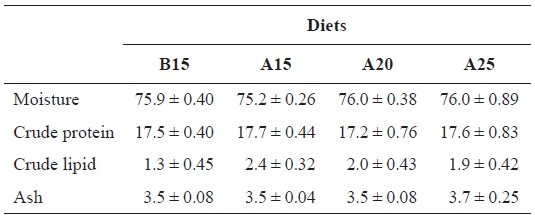
Proximate composition (%) of whole body of juvenile flounder at the end of feeding trial
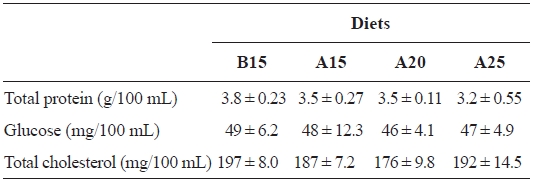
[Table 4.] Hematological changes of the plasma of juvenile flounder at the end of feeding trial
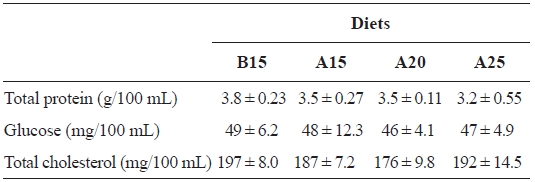
Hematological changes of the plasma of juvenile flounder at the end of feeding trial
flounder was not affected by carbohydrate kind at the same level, but that the feed intake of flounder fed a diet containing β-starch increased compared to that of fish fed a diet contain-ing α-starch. This result was likely due to the low digestibility of β-starch. The feed efficiency and protein efficiency ratio of flounder fed the diet containing α-starch improved compared to that of fish fed the diet containing β-starch. These results are similar to those reported for other fish (Peres and Oliva-Teles, 2002). The relative efficiency of the utilization of di-etary carbohydrates by fish has been associated with the tech-nological treatments applied (Bergot and Breque, 1983) such as the gelatinized ratio of carbohydrates. For example, Jeong et al. (1992) found that rainbow trout fed a diet containing 30% starch exhibited the best overall growth performances when the gelatinized ratio reached 40%. Furthermore, Hilton and Atkinson (1982) observed that the growth performance of rainbow trout decreased when levels of starch comprised more than 14% of the diet. Conversely, other authors have re-ported that higher levels of dietary carbohydrates led to a sig-nificant increase in protein and energy retention (Kaushik and de Oliva-Teles, 1985; Kaushik et al., 1989; Takeuchi et al., 1990). For example, Pieper and Pfeffer (1980) concluded that gelatinized starch was an effective energy source for rainbow trout. Finally, when compared to raw starch, the incorporation of gelatinized starch has been demonstrated to have beneficial effects on the growth and feed efficiency of various fish spe-cies (Furuichi et al., 1987; Takeuchi et al., 1992; Kaushik and Medale, 1994). Thus, the technological treatment of starch de-serves particular attention.
Although the carbohydrate requirements of fish have not been reported, it is the least expensive nutrient in feed for-mulating and can improve the physical properties of extruded and steam pelleted feeds. Therefore, many studies have been focused on optimizing formulation to determine the maximum level of dietary carbohydrates that fish can utilize efficiently. The growth, feed efficiency, and protein efficiency ratios of juvenile olive flounder fed the diet containing 20% α-starch in the present study were higher than those of fish fed the diet containing 15% α-starch. However, the growth of flounder fed the diet containing 25% α-starch decreased compared to that of fish fed the diet containing 20% α-starch, indicating that a dietary inclusion of 25% α-starch would be excessive for the growth of olive flounder. These results suggest that the inclu-sion of 20% α-starch is an optimal level for the growth and feed utilization of flounder. For marine fish species, reports indicate that the dietary incorporation of digestible carbohy-drates should not exceed 20% (Kaushik and de Oliva-Teles, 1985; Beamish and Medland, 1986; Kaushik et al., 1989; Takeuchi et al., 1990; Hemre et al., 1995; Lanari et al., 1999). Conversely, Gouveia et al. (1995) reported that digestible carbohydrates could account for 25% of sea bass diets with no significant effects on growth performance and feed utili-zation efficiency. Additionally, Peres and Oliva-Teles (2002) observed that a dietary incorporation of 25% starch had no negative effects on growth or feed efficiency for European sea bass. In addition, better growth and feed utilization have been reported for rainbow trout fed a diet containing 30% dextrin compared to one that included 8% dextrin (Yamamoto et al., 2000). Finally, Lee et al. (2003) reported that a diet containing 15-25% dextrin was superior to one containing 5% dextrin for flounder.
Providing an adequate dietary carbohydrate level is impor-tant to reduce catabolism of proteins for energy and for the synthesis of glucose, which decreases protein retention and increases nitrogen release to the environment (Suarez and Mommsen, 1987; Cowey and Walton, 1989; Wilson, 1994). The results reported herein indicate that α-starch should ac-count for up to 20% of the diet of juvenile flounder to achieve optimal growth.