In the past, aquaculture was the main industry using micro-algae commercially. Today, various businesses and industries, including those involved in supplementary health products, cosmetics, medicine, and bio-energy, are making extensive use of microalgae (Borowitzka and Borowitzka, 1988).
The advantages of using microalgae as commercial bio-materials include eco-friendly culture methods that allow for continuous reproduction and wide-ranging uses without pol-lutants. However, weaknesses include the sudden death of mi-croalgae, which often leads to high costs and low productivity (Chisti, 2007).
The cost of microalgae used as live food in artificial seed production of shellfish is nearly 30% of the total cost of seed production (Borowitzka, 1997). However, if stable and eco-nomical microalgae production can be developed, microalgae will likely become one of the most important materials in bio-industry.
For mass production of microalgae, reagents used for in-door culture would be inappropriate because of their high cost. Instead, more economical resources, such as agricultural fertilizers, are frequently used (Lopez-Ruiz et al., 1995; Va-lenzuela-Espinoza et al., 2002; Pacheco-Vega and Sanchez-Saavedra, 2009). Using agricultural fertilizers only, however, leads to the problem of lower cell growth rates than in com-mon media such as f/2 (Guillard and Ryther, 1962).
The agricultural fertilizers used in this study were as fol-lows: urea fertilizer containing 46% nitrogen and compound fertilizer (Nam-Hae Chemicals Inc., Yeosu, Korea) contain-ing 22% nitrogen, 12% phosphorus, 17% potassium, and 3% magnesium. The amount of the fertilizers used in this study followed the Schreiber medium standard (Schreiber, 1927) that consists of NaNO3 (100 mg/L) and Na2HPO4?12H2O (20 mg/L). Because 1 L of filtered seawater with 166.7 mg of compound fertilizer and 137.6 mg of urea fertilizer equals the concentrations of nitrate and phosphate in Schreiber medium, this standard was set as 1.0 times the basic fertilizer medium. The fertilizers were ground, dissolved in warm water, and fil-tered immediately before use.
The
>
Culturing N. oceanica with differing concentra-tions of fertilizers and addition of trace elements
To find the optimal concentrations of fertilizers, cell growth was observed for 5 days in the following conditions: 1. f/2 medium as a control group, 2. fertilizer medium 1.0 times (166.7 mg/L of compound fertilizer and 137.6 mg/L of urea fertilizer), 3. fertilizer media 1.25 times (208.4 mg/L of com-pound fertilizer and 172.0 mg/L of urea fertilizer), and 4. fer-tilizer 1.5 times (250.1 mg/L of compound fertilizer, 206.4 mg/L of urea fertilizer).
For trace elements, those used in f/2 medium, such as CoCl2?6H2O (0.110 mg/L), CuSO4?5H2O (0.0196 mg/L), ZnSO4?7H2O (0.044 mg/L), and Na2MoO4?2H2O (0.012 mg/L), were added to the fertilizer medium at varying concentrations: 0.5, 1.0, 1.5, and 2.0 times. The growth rates in these media were observed for 7 days and compared with the growth rate in f/2 medium.
>
Effects of the addition of NaNO3
The concentration of the previous fertilizer medium was reduced to 0.5, 0.25, and 0.17 times. Then, CuSO4?5H2O (0.0588 mg/L) at three times the concentration in f/2 medium and NaNO3 (150 mg/L) at the same concentration as in f/2 medium were added. This experiment involved eight groups, and daily growth in each of the following groups was mea-sured for 7 days: 1) f/2 medium; 2) fertilizer medium 1.25 times; 3) fertilizer medium 0.5 times (compound fertilizer 83.4 mg/L + urea fertilizer 68.8 mg/L + NaNO3); 4) group 3 + CuSO4?5H2O; 5) fertilizer medium 0.25 times (compound fer-tilizer 41.7 mg/L + urea fertilizer 34.4 mg/L + NaNO3; 6) group 5 + CuSO4?5H2O; 7) fertilizer medium 0.17 times (compound fertilizer 28.3 mg/L + urea fertilizer 23.4 mg/L+NaNO3); and 8) group 7 + CuSO4?5H2O.
>
Growth comparison with laboratory and indus-trial reagents
To develop an economical fertilizer medium for the mass production of
Results were analyzed by one-way analysis of variance, and Duncan's multiple range test (Duncan, 1955) was used to detect significant differences at the level of
>
Growth according to concentration of fertilizers and addition of trace elements
The growths of
The results of the 7-day cultures of
centrations of 0.5-2.0 times were as follows. Higher con-tents of trace elements produced higher growth rates of
The growth rates of the experimental groups infused with CuSO4?5H2O and ZnSO4?7H2O were 0.3096-0.3598 and 0.3042-0.3559, respectively. These growth rates were rela-tively high compared with those for other media infused with CoCl2?6H2O or Na2MoO4?2H2O, which showed rates of
0.2903-0.3231.
In the experimental groups infused with either 1.5 (0.0294 mg/L) or 2 times (0.0392 mg/L) CuSO4?5H2O and 2 times ZnSO4?7H2O (0.088 mg/L), the growth rates of 0.3457-0.3598 were still significantly lower than the rate of 0.3726 for the control group in f/2 medium. However, the former groups showed higher growth rates than the rest of the experimental groups (
To test the exact concentration of CuSO4?5H2O for infusion, 2-, 3-, 4-, and 5-fold increased concentrations (0.0392?0.098 mg/L) of CuSO4?5H2O were added to fertilizer medium 1.25 times and cultures were grown for 8 days. The results indicated the significantly highest growth rate of 0.6446 in the control f/2 group (
>
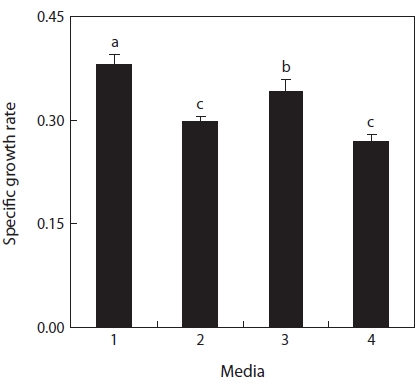
Growth according to addition of NaNO3
On the basis of the findings in this study, growth differ-ences in f/2 and fertilizer media seemed to be correlated with the high content of ammonia and low content of nitrate. With the infusion of CuSO4?5H2O (0.0588 mg/L) and NaNO3 (150 mg/L), the amounts of fertilizers were reduced by 0.5 times (compound fertilizer, 83.4 mg/L; urea fertilizer, 68.8 mg/L), 0.25 times (compound fertilizer, 41.7 mg/L; urea fertilizer, 34.4 mg/L), and 0.17 times (compound fertilizer, 28.3 mg/L;
urea fertilizer, 23.4 mg/L) the level in Schreiber culture me-dium. As a result (Fig. 4), the growth rate of 0.3985 in fertil-izer medium 1.25 times was significantly the lowest rate (
>
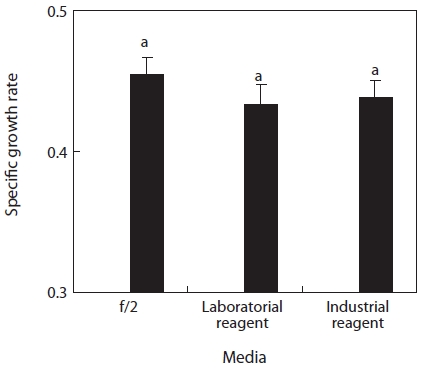
Growth rate comparison between laboratory and industrial reagents
From the previously mentioned fertilizer media 0.25 times (compound fertilizer, 41.7 mg/L + urea fertilizer 34.4 mg/L) infused with CuSO4?5H2O 0.0588 mg/L and NaNO3 150 mg/L, NaNO3 and CuSO4?5H2O were assessed separately with labo-ratory and industrial reagents. The growth rates of
Culture media for microalgae should be economical, allow for high growth rates, satisfy the needs of the microalgae, and be easy to prepare. F/2 medium, the most commonly used me-dium for small-scale indoor culture, is costly and difficult to set up for outdoor mass culture (Fabregas et al., 1985). Thus, agricultural fertilizers are commonly used as a replacement for f/2 culture medium (Fabregas et al., 1987; Bae, 2004; Pache-co-Vega and Sanchez-Saavedra, 2009). However, cell growth rates in such fertilizer-based media have not yet reached that in f/2 culture medium. The slower rate is attributable to the presence of nitrogen and phosphorus, major components in fertilizer cultures (Ukeles, 1980; Gonzalez-Rodriguez and Maestrini, 1984; Bae, 2004). Lack of trace elements and vita-mins necessary for the growth of microalgae are also reasons for lower growth rates (Stein, 1973).
Our aim was to develop media using economical and conve-nient agricultural fertilizers to replace f/2 medium for outdoor mass culture of
From the second and third days after inoculation, the growth stage of the cells was in log phase in f/2 media. On the other hand, fertilizer media showed a longer lasting lag phase, and the lower level of the ultimate highest cell den-sity was problematic. The following factors are believed to have caused such results: urea fertilizer, consisting of 46% nitrogen, and compound fertilizer consisting of 22% nitrogen, 12% phosphorus, 17% potassium, and 3% magnesium. Other causes may have been the lack of essential trace elements for the growth of
After adding the four trace elements (Co, Cu, Zn, Mo) used in f/2 medium to each fertilizer medium, growth rates of
Gonzalez-Rodriguez and Maestrini (1984) used 12 kinds of fertilizers for 16 microalgal species with Conway medium as a control (Walne, 1966). The result of their research, which was similar to our result, showed extremely low growth rates of
Bae (2004) cultured
In this study, the growth of microalgae in the fertilizer me-dia was slower than that in f/2 medium in the early stages of the experiment. The low level of the highest cell density was also believed to be due to the high ammonium content.
Thus, to reduce the concentration of ammonia in the fertil-izer media, the amounts of fertilizers were reduced to 25% of Schreiber’s nitrate and phosphate concentrations. The growth of
In conclusion, for 1 ton of filtered seawater, an optimal me-dium for the mass culturing of
![Specific growth rate of Nannochloropsis oceanica cultured with agricultural fertilizer media added with different concentrations of trace elements [1 f/2; 2 compound fertilizer 208.4 mg/L + urea fertilizer 172.0 mg/L; 3 2 + CoCl2 (0.055 mg/L); 4 2 + CoCl2 (0.110 mg/L); 5 2 + CoCl2 (0.165 mg/L); 6 2 + CoCl2 (0.220 mg/L); 7 2 + CuSO4?5H2O (0.0098 mg/L); 8 2 + CuSO4?5H2O (0.0196 mg/L); 9 2 + CuSO4?5H2O (0.0294 mg/L); 10 2 + CuSO4?5H2O (0.0392 mg/L); 11 2 + ZnSO4?7H2O (0.022 mg/L); 12 2 + ZnSO4?7H2O (0.044 mg/L); 13 2 + ZnSO4?7H2O (0.066 mg/L); 14 2 + ZnSO4??7H2O (0.088 mg/L); 15 2 + Na2MoO4?2H2O (0.006 mg/L); 16 2 + Na2MoO4?2H2O (0.012 mg/L); 17 2 + Na2MoO4?2H2O (0.018 mg/L); 18 2 + Na2MoO4?2H2O (0.024 mg/L)].](http://oak.go.kr/repository/journal/11587/E1HKAL_2011_v14n4_317_f002.jpg)
![Specific growth rate of Nannochloropsis oceanica cultured with agricultural fertilizer media added with different concentrations of CuSO4 5H2O [1 f/2; 2 compound fertilizer 208.4 mg/L + urea fertilizer 172.0 mg/L; 3 2 + CuSO4 5H2O (0.0392 mg/L); 4 2 + CuSO4?5H2O (0.0588 mg/L); 5 2 + CuSO4?5H2O (0.0784 mg/L); 6 2 + CuSO4 5H2O (0.098 mg/L)].](http://oak.go.kr/repository/journal/11587/E1HKAL_2011_v14n4_317_f003.jpg)
![Specific growth rate of Nannochloropsis oceanica cultured with low concentration of agricultural fertilizer media added with NaNO3 (150 mg/L) and CuSO4 (0.0588 mg/L) [1 f/2; 2 compound fertilizer 208.4 mg/L + urea fertilizer 172.0 mg/L; 3 compound fertilizer 83.4 mg/L + urea fertilizer 68.8 mg/L + NaNO3; 4 3 + CuSO4?5H2O; 5 compound fertilizer 41.7 mg/L + urea fertilizer 34.4 mg/L + NaNO3; 6 5 + CuSO4?5H2O; 7 compound fertilizer 28.3 mg/L + urea fertilizer 23.4 mg/L + NaNO3; 8 7 + CuSO4?5H2O].](http://oak.go.kr/repository/journal/11587/E1HKAL_2011_v14n4_317_f004.jpg)