To find seasonally optimal microalgae for mass culture of the rotifer Brachionus plicatilis, the growth rates of 12 microalgal spe-cies (two marine Chlorella spp., five marine Nannochloris spp., two marine Nannochloropsis spp., one estuarine Nannochloropsis sp., and two estuarine Chlorella spp.) were compared at 25℃ at 15 psu and 30 psu. Among these, six species showing high growth rates were chosen and examined again at high (30℃ and 32℃) and low (10℃) temperatures. Their amino and fatty acids and the dietary value of the rotifers that fed on each microalgal species were examined. Nannochloris sp. (KMMCC-119) and Chlorella vulgaris (KMMCC-120) showed the highest growth rates at temperatures over 30℃ and at 10℃, respectively. The growth rate of Nannochloris was higher than those of Chlorella and Nannochloropsis at high temperatures, but lower than those of the latter at low temperatures. The growth rate of rotifers fed on Nannochloropsis was highest and that of those fed on Chlorella was lowest. Levels of eicosapentaenoic acid and docosahexaenoic acid were highest in Nannochloropsis and lowest in Nannochloris. However, total amino acid content was highest in Nannochloris and lowest in Chlorella. In conclusion, Nannochloropsis sp. (KMMCC-33) was the best microalgal species for the mass culture of the rotifer. However, during high- or low-temperature seasons in which Nannochloropsis does not grow well, Nannochloris spp. (KMMCC-119, 395) and C. vulgaris (KMMCC-120) would adequately replace Nannochloropsis sp. (KMMCC-33).
The rotifer
Examples of commonly used microalgae and yeast include the microalgae
Yeasts are more economical than microalgae, but contain insufficient amounts of unsaturated fatty acids, which are es-sential for the growth of rotifers (Watanabe et al., 1980; Kim et al., 2009). In an attempt to supplement the shortcomings of yeast, ω-yeast was developed using bread yeast infused with squid oil. However, this had lower nutritional value than microalgae and caused water pollution (Hirayama and Funa-moto, 1983). Thus, in spite of the high costs incurred on the mass culture of rotifers, live microalgae are still preferred by hatcheries (Hur, 1991; Borowitzka, 1997).
Therefore, this study aimed to identify a new species among
The microalgae used in this study, from the Korea Marine Microalgae Culture Center (KMMCC), included nine ma-rine species and three estuarine species. These consisted of four kinds of
Microalgae in the log phase stage were inoculated in 100 mL of f/2 culture medium (Guillard and Ryther, 1962) in a 250-mL Ehrenmeyer flask at a density of 100 × 104 cells/mL. Microalgae were cultured in standing water at 25℃ and 15 and 30 psu, with continuous light of 100 ㎛ol m-2 s-1, three times during 7 days. The salinity was adjusted by the mixture
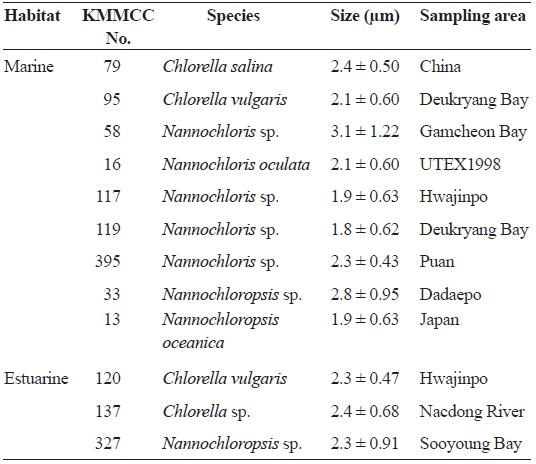
[Table 1.] List of microalgal species used in the study
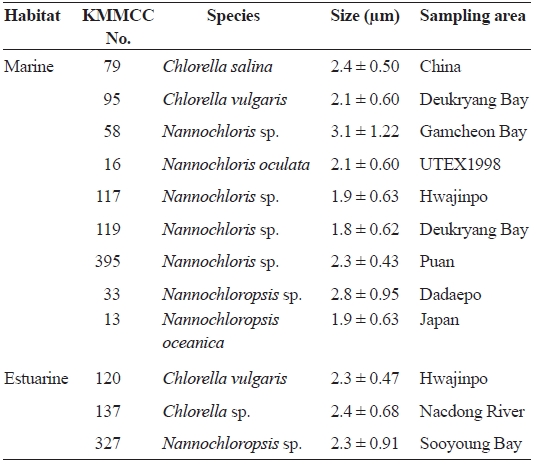
List of microalgal species used in the study
of filtered seawater and distilled water.
To measure growth rate, cells were counted by hemacy-tometer regularly, four times a day. The specific growth rate (SGR) was calculated according to Guillard (1973) [SGR = 3.322 × log(N1/N0)/t, where t is culture days after inocula-tion, and N0 and N1 are cell density after inoculation or t days, respectively].
>
Growth of six kinds of microalgae at high and low temperature
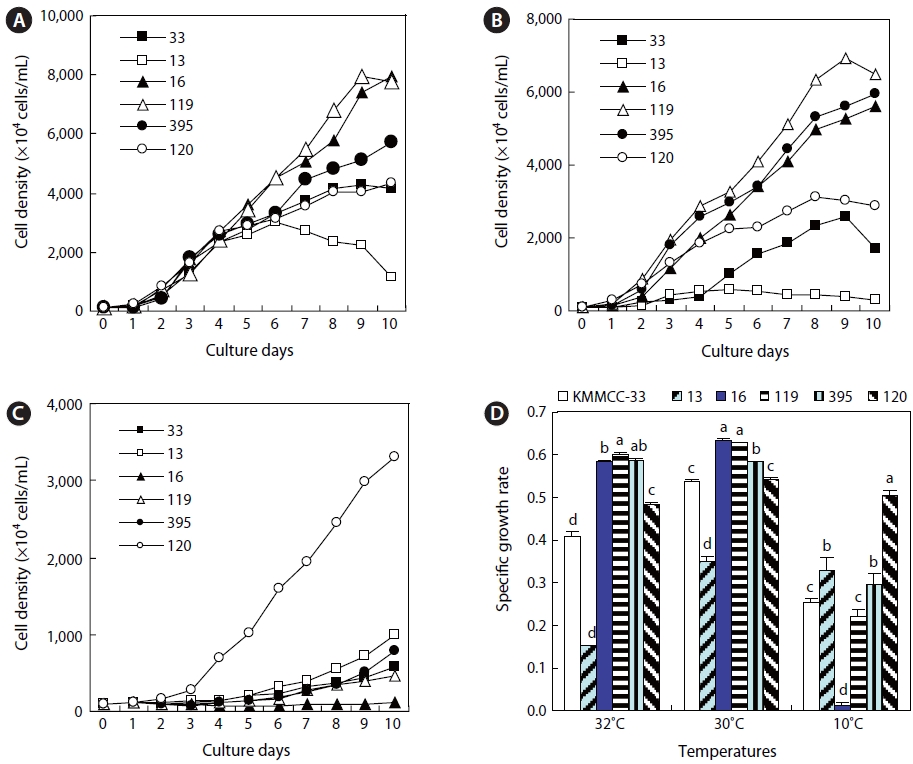
Six kinds of microalgae that showed high growth rates in the aforementioned experiment were cultured at high tempera-tures of 30℃ and 32℃ and a low temperature of 10℃ at 15 psu with 100 ㎛ol m-2 s-1 of continuous lighting, namely,
>
The adequacy of the six microalgae as rotifer feed
The rotifer
>
Analysis of the nutritional content of the microal-gae and rotifers
The amino and fatty acids of the six selected microalgae and the rotifers fed on three selected microalgae, which in-duced high growth rates in rotifers, were analyzed. They were cultured by the method described above. At the end of the log phase of growth, they were harvested and kept at -80℃ until analysis.
For the analysis of amino acids, 20 mg of sample infused with 15 mL of 6 N HCl was heated, sealed, and hydrolyzed at 110℃ for 24 h. The sample was then filtered and dried to remove HCl. Then 25 mL of the sample was set by sodium dilution buffer (pH 2.2) and a portion of the sample was ana-lyzed by the ninhydrin method using S433 (Sykam, Fursten-feldbruck, Germany). Conditions of the analysis were as fol-lows: column size, 4 mm × 150 mm; absorbance level, 570 nm and 440 nm; reagent flow rate, 0.25 mL/min; buffer flow rate, 0.45 mL/min; reactor temperature, 120℃; reactor size, 15 m; and analysis time, 65 min.
For the analysis of fatty acids, 20 mg of sample in a 15-mL flask was added to 2 mL of 10% BF3-methanol. Nitrogen was added to the sample and heated at 85℃ for an hour and a half to draw methyl ester (Morrison and Smith, 1964; Budge, 1999). Cooled to 30-40℃, the sample was combined with wa-ter and hexane to draw fatty acids separately. The extracted fatty acids were analyzed with a HP GC 6890 Plus installed with a HP autosampler (Agilent Technologies, Santa Clara, CA, USA). The GLC used in this analysis was the DB-225 (20 m × 0.1 mm, i.d., 0.1 ㎛ film thickness; J&W Scientific, Agilent Technologies, Santa Clara, CA, USA). Conditions of the analysis were as follows: column temperature levels, 60-195℃ (25℃/min); temperature conditions, 195-205℃ (3℃/min), 205-230℃ (8℃ min); injector, 250℃; detector, 250℃; and carrier gas used, He (60 cm/s). Fatty acids were identified by comparison with known standards.
The results of this study were analyzed by one-way ANO-VA, and Duncan's multiple range test (Duncan, 1955) was ap-plied for the significance level (
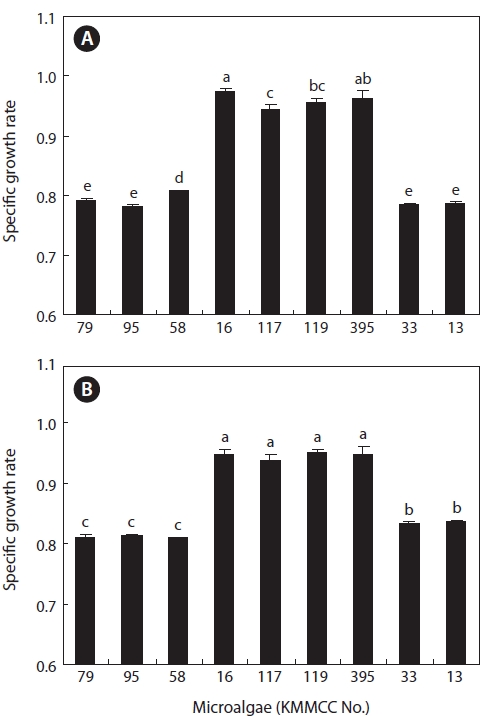
After 7 days culture of the nine marine microalgae at 30 psu and 15 psu, results (Fig. 1) indicated that
At 15 psu, the growths of
the range of 0.9393 and 0.9504 (highest cell density, 9,718 × 104 cells/mL to 10,145 × 104 cells/mL). Conversely,
>
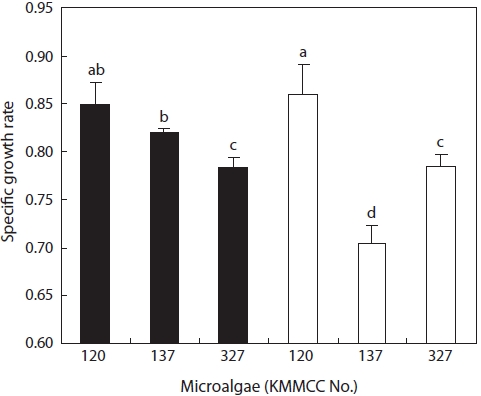
Growth of estuarine microalgae
At 30 and 15 psu, the growth rates of
nificantly highest rate (
In contrast to the estuarine
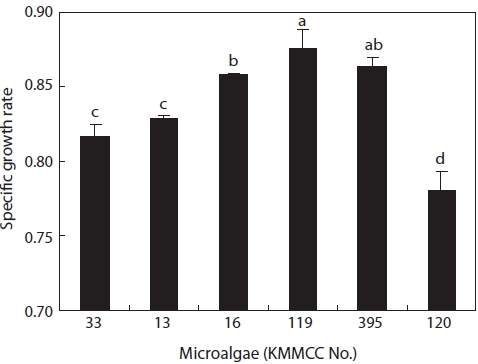
The growth and nutritional content of the marine microal-gae
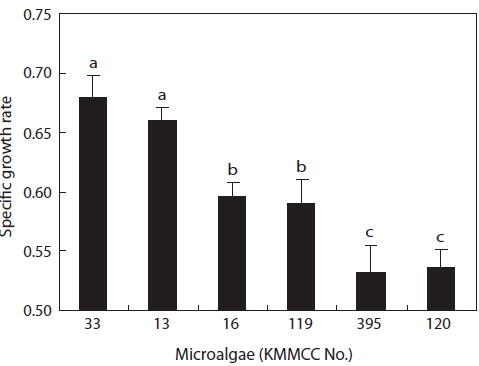
Growth rates under the same culture conditions, 15 psu, 25℃, and 100 ㎛ol m-2 s-1, are shown in Fig. 3. Among the six kinds of microalgae, the growth rate of
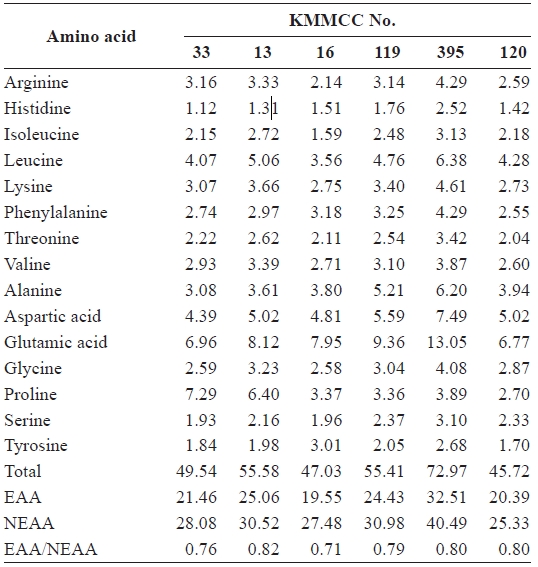
[Table 2.] Amino acid composition (%) of six microalgal species
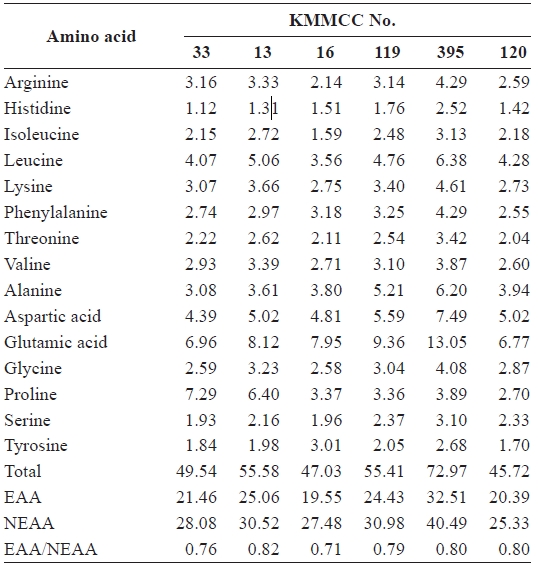
Amino acid composition (%) of six microalgal species
higher than that of
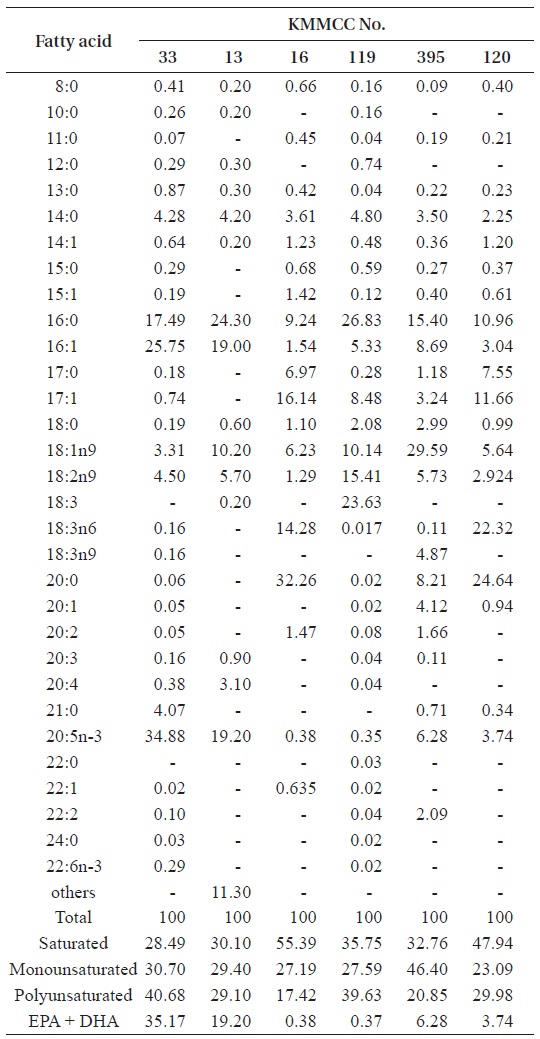
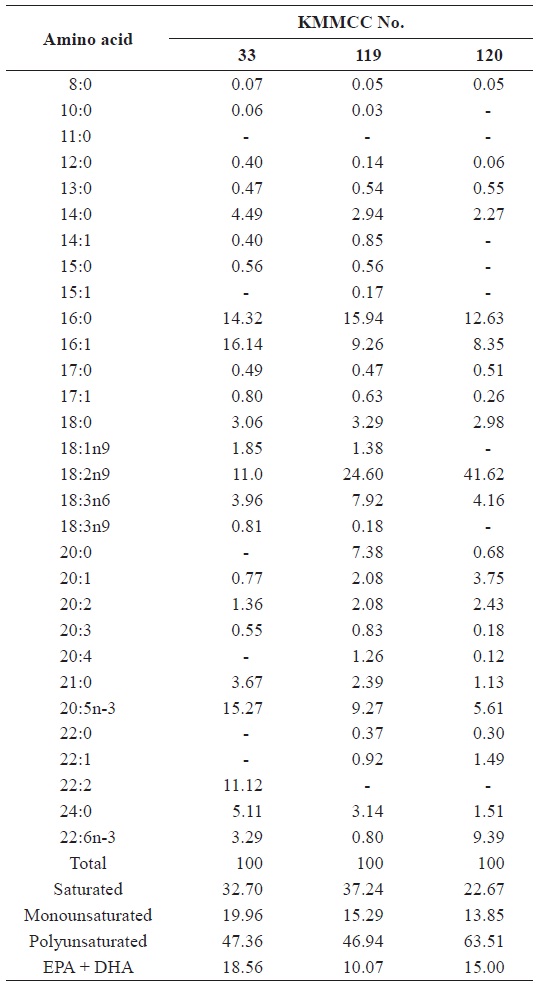
[Table 3.] Fatty acid composition (%) of six microalgal species
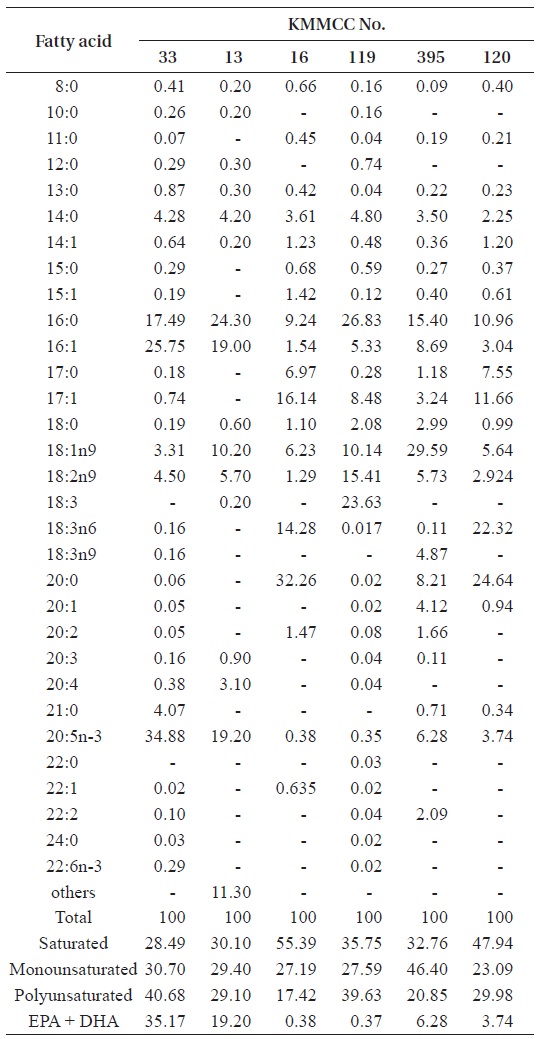
Fatty acid composition (%) of six microalgal species
20:5n-3) in
>
Growth of Nannochloropsis, Nannochloris, and Chlorella in high and low water temperatures
The growth rates of six kinds of
The growth rates of
At 10℃, the growth rate of
The growth rates of rotifers fed on the six kinds of microal-gae are shown in Fig. 5. In 5 days of culture, the growth rates of rotifers fed on
For the contents of fatty acids in rotifers fed on the afore-mentioned three kinds of microalgae (Table 5), those fed on
The growth of rotifers depends on the kind of microalgae used as feed (Hirayama et al., 1979; Cho et al., 2007). Various kinds of microalgae as feed for rotifers have been reported, with
One of the obstacles to the mass culture of rotifers comes from difficulties in the outdoor mass culture of microalgae during certain seasons. In summer, sudden cell mortality of-ten occurs, and in the winter, the cell growth rates tend to be very low. Thus, further developing microalgae that are highly adaptive to conditions in the two aforementioned seasons is
[Table 4.] Amino acid composition (%) of Brachionus plicatilis fed different microalgal species
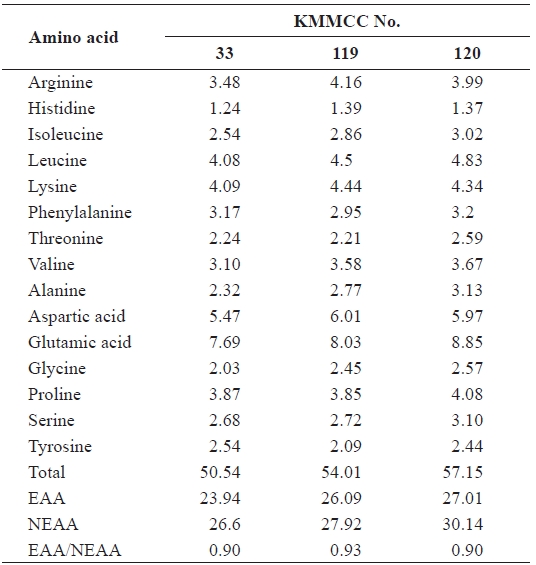
Amino acid composition (%) of Brachionus plicatilis fed different microalgal species
essential (Watanabe et al., 1978; James and Abu-Rezeq, 1988; Hur, 1991).
This study aimed to identify microalgae among
Nine kinds of marine microalgae showed similar growth rates to each other at 15 and 30 psu. Their growth, howev-er, exhibited slight differences according to microalgal type
[Table 5.] Fatty acid composition (%) of Brachionus plicatilis fed different microalgal species
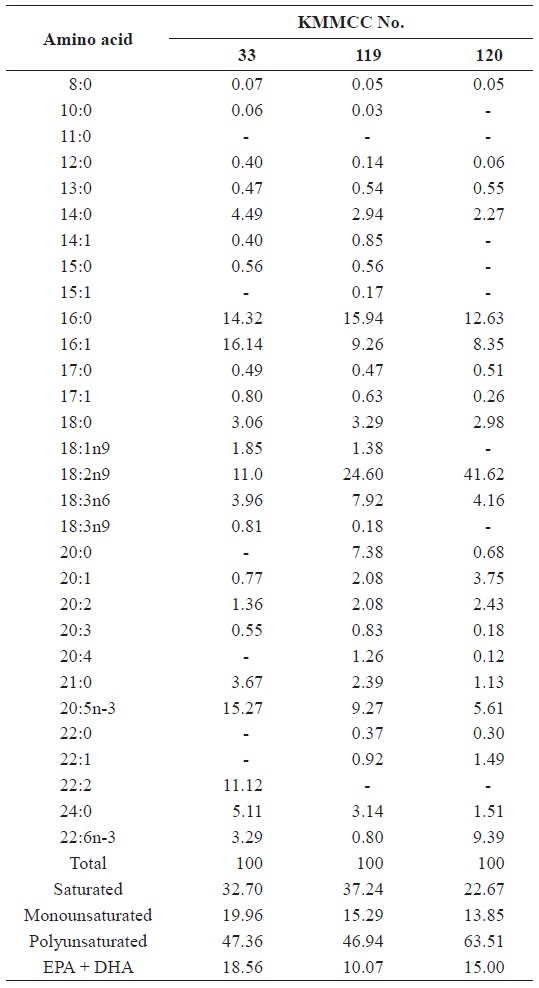
Fatty acid composition (%) of Brachionus plicatilis fed different microalgal species
and salinity. Three estuarine microalgae also showed similar growth rates at 15 and 30 psu. Based on these results, the 12 kinds of microalgae studied in this research can be inferred to be suitable as feed for rotifers because they were euryhaline.
The growth rates of the six kinds of microalgae cultured at 30℃ and 32℃ for the high-temperature experiments were highest for
Among the three kinds of
At 10℃, for the low-temperature experiment,
At high temperature,
The essential amino acid contents of most microalgae are in-fluenced by factors including the intensity of lighting (Thomp-son et al., 1990; Brown et al., 1997), temperature (James et al., 1989; Thompson et al., 1992), pH (Guckert and Cooksey, 1990), culture medium (Wikfors et al., 1984), and harvesting times (Brown et al., 1997; Pernet et al., 2003). In this study, the fatty acid contents of the two kinds of
With regard to EPA and DHA, Volkman et al. (1993) re-ported that
The fatty acid contents of rotifers fed on
In conclusion,