Although culture techniques for the abalone Haliotis discus hannai are well known, mass culture of the benthic microalgae that are essential live food for the abalone larvae is still not practiced. This study was conducted to identify the microalgal species suit-able for the growth of early larvae of H. discus hannai. The growth and attachment rates of 31 microalgal species were examined. Acrylic plates were used as the substrate. Among the 31 microalgal species, nine showing high growth and attachment rates were selected and tested for their dietary values via factors including settlement, metamorphosis, and survival rates of abalone larvae. Tetraselmis hazeni and Rhaphoneis sp. induced the highest settlement rate (65-69%) in abalone larvae. The metamorphosis rate was highest (57%) in larvae fed Rhaphoneis sp. and was also significantly higher in larvae fed Oscillatoria splendida (29%) and T. hazeni (22%) than in those fed other species. The highest survival rate of the larvae during the 15 days after metamorphosis was 67% in those fed Rhaphoneis sp., followed by T. hazeni (42%) and O. splendida (35%). In conclusion, Rhaphoneis sp. is the most suitable diatom for use as a live food for the culture of early larvae of H. discus hannai. In addition, T. hazeni and O. splendida are also potential species to be further developed and utilized in larval culture.
Biofilm composed of mucus plays an important role in the settlement processes of invertebrate larvae such as
Larval growth differs depending on the microalgal charac-teristics, and food resources for larvae may become insuffi-cient when large amounts of microalgae fall off the acrylic plate due to their weight. When the microalgal membrane is flat, larval settlement onto the biofilm tends to be successful. However, if the membrane is three-dimensional, larvae show low settlement rates (Kawamura and Kikuchi, 1992; Searcy-Bernal, 1996; Roberts et al., 2007a). Furthermore, some dia-toms with hard silica cell walls tend to have low digestion rates by the larvae. Therefore, it is necessary to obtain high-quality microalgae attached to acrylic plates to achieve the successful artificial seed production of
Studies on the cultivation of early and immediate post-set-tlement larval stages are extremely limited compared to those of later larval stages. Moreover, studies on the effectiveness of feed for the larvae of
>
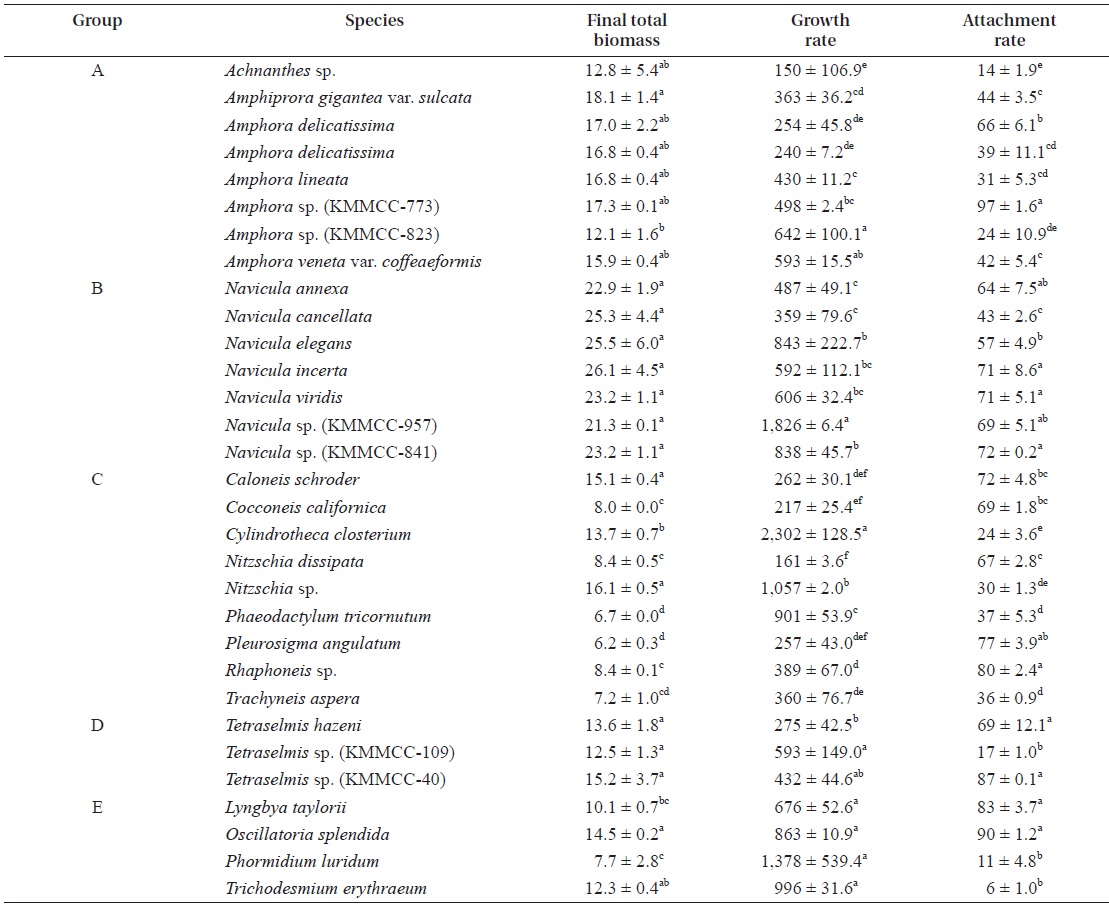
Growth and attachment rates of microalgae
The growth and attachment rates of 31 kinds of microalgae selected from the Korea Marine Microalgae Culture Center (KMMCC), including 24 diatoms, three green algae
[Table 1.] List of microalgal species for the study
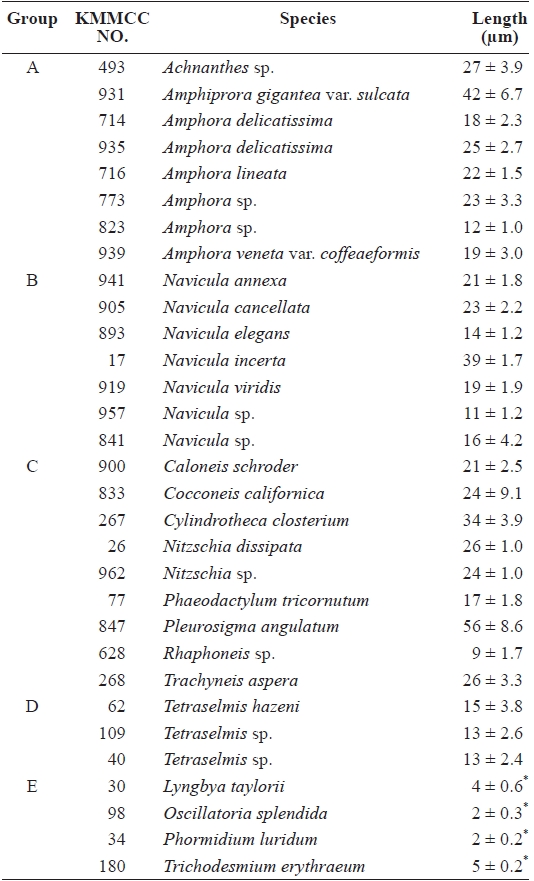
List of microalgal species for the study
continuous lighting.
Two weeks later, floating microalgae were collected us-ing GF/C filters. Attached microalgae were detached from surfaces of the substratum using a soft brush and filtered by GF/C filters. The collected microalgae from each tank were separately dried at 60℃ for 2 h and weighed to the nearest milligram. The following formulae were used for calculating the attachment and growth rates of the microalgae:
Attachment rate (%) = (Weight of attached microalgae/Total weight of microalgae) × 100
Growth rate (%) = [(n1 - n0)/n0 × 100 (n1: final collection amount (mg), n0: initial inoculation amount (mg)]
>
Settlement, metamorphosis, and survival rates of Haliotis discus hannai
Larvae of the veliger stage were examined 56 h after fer-tilization at 21℃ and were used in the present study. Nine species among the 31 microalgae showed higher growth and adhesion rates. To culture these species, 10 mL of f/2 medium was placed inside a 6-well tissue culture chamber, and the full-grown microalgae were inoculated at the 10% level. They were cultured without aeration at 20℃ and 40 ㎛ol m-2s-1 of constant light for 10 days.
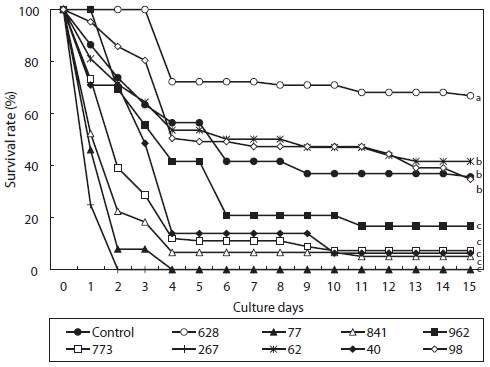
Each chamber in which the microalgae had been cul-tured was used for larval culture. Before larval culture, the chambers were cleaned several times with filtered seawater to remove the f/2 medium. Then, 30 larvae were placed into the chamber filled with 10 mL of filtered seawater. The cell density of the microalgae attached to the chamber was ap-proximately 5-10 × 104 cells/mL. The larvae were reared at 20℃ and 40 ㎛ol m-2s-1 under a photoperiod of 8:16 h LD (light:dark) for 15 days. Upon the completion of larval settle-ment onto the microalgae, the culture water in the chamber was exchanged with 5 mL of filtered seawater daily. The con-trol group contained no microalgae and was filled only with filtered seawater. All experiments were repeated six times for each treatment. The settlement and metamorphosis of the lar-vae were observed through a dissecting microscope. Larvae attaching to the microalgal film and having a foot were consid-ered to be settled larvae and larvae with a shell molding were considered to be metamorphosed larvae. Larvae that showed no metamorphosis for 6 days were eliminated. The settlement rate measurement was repeated seven times. Measurements of metamorphosis rates and survival rates after metamorphosis were repeated twice. Dead larvae, immobile for more than 10 seconds with no heartbeat, were removed daily. Survival rates after metamorphosis were measured.
Settlement rate (%) = (No. of settled individuals/No. of in-oculated individuals) × 100
Metamorphosis rate (%) = (No. of metamorphosed individ-uals /No. of settled individuals) × 100
Survival rate (%) = (No. of surviving individuals/No. of metamorphosed individuals) × 100
Data were analyzed by one-way analysis of variance (ANOVA), and Duncan’s multiple range test (Duncan, 1955) was applied for the significance level (
>
Growth and attachment rates of microalgae
The growth and attachment rates of each microalgal group
[Table 2.] Final total biomass (mg) and growth and attachment rate (%) of microalgal species

Final total biomass (mg) and growth and attachment rate (%) of microalgal species
were examined (Table 2). There was no significant differ-ence in diatom biomass within group A, except for
The microalgal biomass of
The biomasses of the diatoms
The biomasses of three kinds of
The biomasses of
>
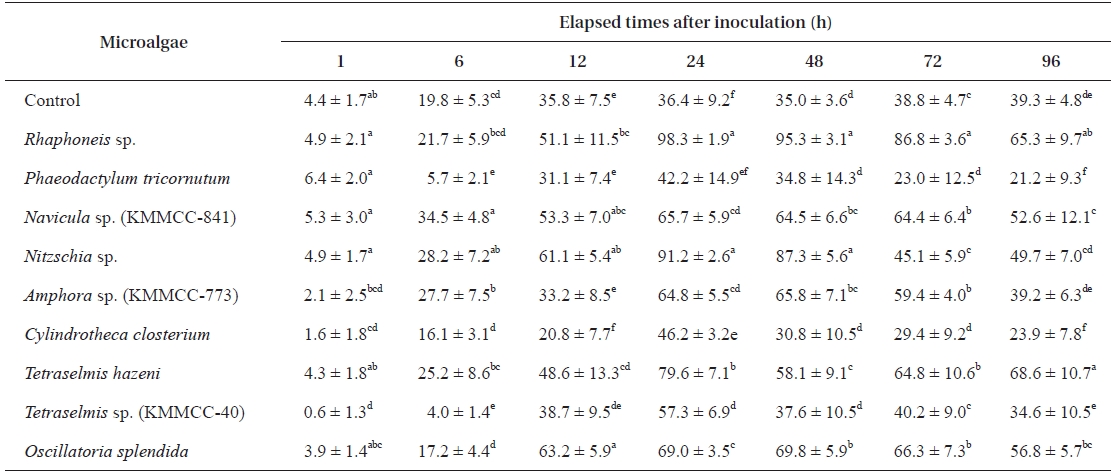
Settlement, metamorphosis, and survival rates of Haliotis discus hannai larvae
Nine microalgal species showing high growth and attach-ment rates were examined for their effectiveness as feed for
[Table 3.] Settlement rate (%) of Haliotis discus hannai larvae on different microalgal species
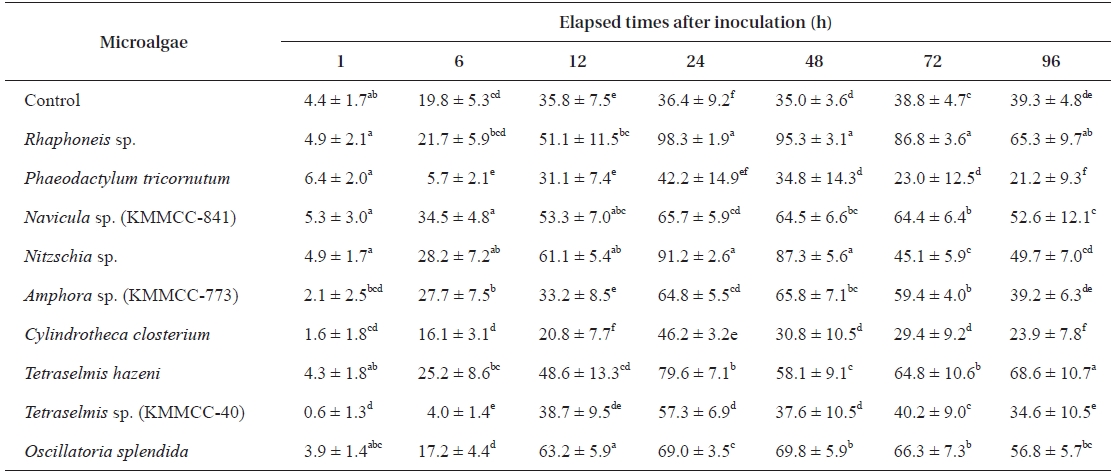
Settlement rate (%) of Haliotis discus hannai larvae on different microalgal species
reached their maxima after another 12 h. After 24 h, the larvae showed a tendency to constantly perish (Table 3).
After 24 h, the settlement rates in all experimental groups except for
The metamorphosis rates of the larvae that fed on
The experimental group fed on
The larvae of
Diatoms generally have high attachment rates compared to other microalgae, but most microalgae can adhere to substrate to a certain degree. In terms of nutritional value, diatoms, green algae, and blue-green algae generally have high con-tents of lipid, protein, and minerals, respectively (Borowitzka and Borowitzka, 1988). Diatoms can be divided into eight types in terms of the existence of mucous, their attachment form, and mobility. Among them, the best types as feed for the larvae of
In this study, diatoms generally showed higher attachment rates than did other microalgae. However,
With respect to the settlement and metamorphosis of the abalone larvae,
Within two days after metamorphosis, the larvae of
However, immediately following metamorphosis, the lar-vae receive nutritional substances from yolk. Thus, it is dif-ficult to observe differences in their growth according to feed type during such early developmental stages (Kawamura et al., 1998). Moreover, the premature development of the diges-tive system of the larvae at early stages makes it hard to ex-amine the effectiveness of feed (Roberts et al., 1999). For the larvae of
In this study, larvae which fed on microalgae except for
The diameter of the larval mouth after metamorphosis mea-sures only 10 ㎛; thus, the size of microalgae is also important as a condition of suitable feed for abalone larvae (Kawamura et al., 1998). The sizes of microalgae used in this study, except for
In this study, green algae and blue-green algae other than diatoms were also found to be prospective feeds for the larvae of