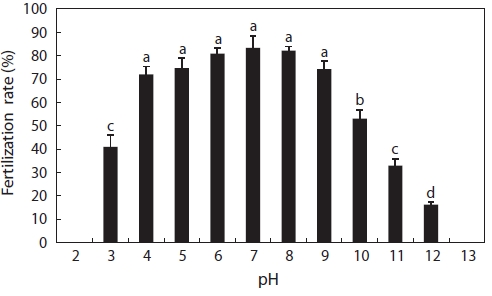
In this experiment, we examined the survival, fertilization, hatching times, and hatching rates of Far Eastern catfish Silurus asotus at pH ranging from 2 to 13 under laboratory conditions. Eggs could be fertilized at pH 3-12. In a hatching experiment, mortality was first observed at pH 13, when all fertilized eggs died within 8 min, followed by pH 2 (30 min), pH 12 (60 min), pH 3 (4 h), and pH 11 (5 h). Hatching only occurred at pH 4-10, with the highest hatching rate at pH 7 (52%) and the lowest at pH 10 (24%). Hatching rates in acid solutions were higher than in alkaline solutions, although the difference was not significant. Hatching was first observed at pH 10, beginning 27 h after fertilization and ending at the 31 h. A clear difference was observed between hatching times, ranging from 31 to 64 h and increasing in order with descending pH.
pH is the main factor affecting water quality. Extreme pH negatively affects fish growth and reproduction (Zweig et al., 1999) and even causes massive mortalities in fish culture. Sensitivity to extreme pH conditions varies according to fish species and age, with fish showing lower tolerance at the em-bryonic and larval stages (Lloyd and Jordan, 1964). The fertil-ization of most fish species is unsuccessful at water pH lower than 4.0 (Peterson et al., 1982). The fertilization success of Atlantic salmon
Generally, the effect of low pH on fish fertilization and hatching is not often a subject of research (Sayer et al., 1993) except in salmonids. Many studies have been made on the ef-fects of acidification on the fertilization and hatching of salm-on and trout, but such studies on Far Eastern catfish
The water used was groundwater with a pH of 7.34, which also served as the control water. The study was conducted at the aqua farm of Kunsan National University. pH solutions ranging from 2 to 13 were adjusted using sodium hydroxide and hydrochloric acid. Five milliliters of pH solutions was added to Petri dishes for the observation of fertilization. For the hatching experiment, 200 mL of pH solution was added to a 400-mL beaker, and solutions were aerated with an air pump to ensure sufficient dissolved oxygen (above 7 mg/L). Temperature was maintained at 23.5 ± 1℃ and pH was mea-sured with a Mettler Toledo MP-220 (Mettler-Toledo GmbH, Switzerland). pH was tuned every hour and the solutions were wholly changed every 6 h during all experimental periods. Fertilization and hatching experiments were performed in trip-licate for each pH level.
The experiment was performed at the aqua farm of Kunsan National University. Three-year-old brood fish were selected and kept isolated in holding tanks. Spawning was induced in female brood fish using intraperitoneal injections of hu-man chorionic gonadotropin at the rate of 10,000 IU/kg body weight. These fish were then stocked for 12 h at a water tem-perature of 28 ± 0.5 ℃. Twelve hours later, eggs were stripped into a dry bowl. Sperm were obtained through surgical remov-al of the testis. Adherent tissue was dissected away and the tes-tes were pooled in physiological saline. Some of the stripped eggs were fertilized immediately with milt after sperm activa-tion was initiated by the addition of water and stocked in an incubator. Sperm from one male were used to fertilize eggs from one female. The remaining eggs were stocked in a dry bowl and sperm were stocked in physiological saline for the fertilization experiment.
>
Fertilization and hatching rates
To observe the fertilization rate, 50 eggs were placed into the prepared 5 mL pH solutions; sperm were injected into the dish and mixed equably, and after about 1 min of gentle stirring, fertilization could be checked under a dissection microscope (10~100× magnification). Fertilization membranes formed immediately when an egg was fertilized so we could use them as a standard to determine whether such an event occurred. Fertilization rates were calculated as the number of total eggs divided by the number of fertilized eggs.
To determine the hatching rate, 50 fertilized eggs were se-lected from the incubator and placed randomly into the 200 mL prepared pH solutions. Mortalities and hatching rates were then recorded during the experimental period. Eggs were considered dead when parts of the content turned opaque and white. Dead eggs were counted and removed to prevent fungal growth. Hatching was defined as the rupture of the egg mem-branes by the tail. The hatching rate was determined as the proportion of hatched eggs to total eggs. Hatching time was recorded as the time span between fertilization and the hatch-ing of the last egg.
Data were analyzed using SPSS version 16.0 (SPSS Inc., Chicago, IL, USA). One-way analysis of variance (ANOVA) was used to compare the mean values at different pH levels.
In this experiment, catfish eggs were fertilized at pH 3-12, as shown in Fig. 1. Fertilization rates from pH 4 to 9 were above 70% and obviously higher than those at pH 3, 10, 11, and 12. The fertilization rates generally declined with decreas-ing acid concentra-tions and increasing alkalinity concentrations. No fertilization occurred at pH 2 or 13. The decrease in fertilization success may have been due to the effects of acidic water either on spermatozoa motility or on the eggs. We ob-served that egg membranes dissolved at pH 2, although at pH 13, eggs became opaque and white in a few minutes; we, how-ever, did not notice the motility of spermatozoa. According to Daye and Glebe (1984), the motility time of Atlantic salmon spermatozoa decreased from pH 7.0 to 4.0, around which no motility was found after a lag of 20 s. The proportion of motile spermatozoa of brook trout decreased as the pH was reduced from 5.0 to 3.5 and completely ceased at pH 3 (St-Pierre and Moreau, 1987). Similar findings were reported for alkaline conditions. The sperm of common carp had a lower period of motility when the pH value of the water was raised to be-tween 8.2 and 9.5, and pH values above 9.0 were found to be lethal (Elster and Mann, 1950). In our experiment, eggs could be fertilized at pH 3-12. This was a considerably wide range compared to other fish species, suggesting that catfish eggs were more tolerant of extreme pH conditions. However, in pH 12, 11, and 3 solutions, the fertilized eggs did not survive long, all dying within 1 h, 5 h, and 4 h, respectively.
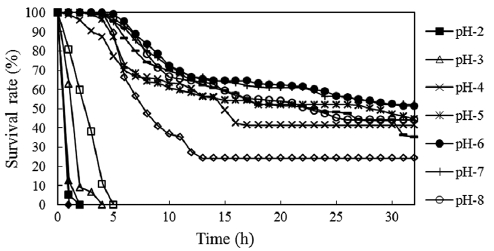
The survival rates of fertilized eggs at different pH levels are shown in Fig. 2. Mortality was first observed in pH 13 so-lutions, with all eggs dying within 8 min. At pH 2, embryonic
development stopped completely and all eggs died within 30 min. Cleavage occurred at pH 12 and some eggs developed to the 2-cell stage, but all eggs died in 1 h. Eggs started dying 60 min after fertilization in pH 3 solutions. Two-thirds of the eggs died before entering the 4-cell stage, and all eggs died within 4 h. At pH 11, most eggs died before the morula stage and all died within 5 h postfertilization. Mortality was high in the first 15 h in these solutions, after which time the death rate became low. The period of sensitivity to acidity and alkalin-ity appeared to be the first 5 h after fertilization. This phe-nomenon has also been reported for other species of fish such as
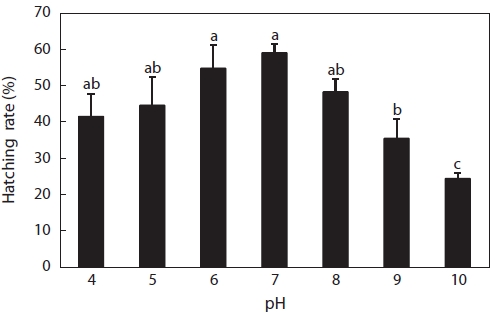
Hatching rates are shown in Fig. 3. Hatching occurred at pH 4-10, but the hatching rate was low, even in the control with the highest hatching rate of 52%, followed by pH 6, pH 5, pH 8, pH 4, pH 9, and pH 10. No obvious difference was seen between the control and pH 4, 5, 6, or 8 solutions, but control rates were significantly higher than those in pH 9 and 10 solu-tions. Hatching rates in acid solutions were higher than those in alkaline solutions. In fact, this was supposed to be a wide pH range for hatching compared to other fish species. Many reports of hatching failure under such acidic conditions have been published. For example, Trojnar (1977) reported that de-formations and death of white sucker
However, fewer reports have described the tolerance of fish to alkaline solutions, and generally, the pH range of 5-9 is ac-cepted as harmless to fish. Krishna (1953) found that for trout eggs and alevins, mortalities occurred above a pH value of 9.0, but the length of exposure was not given. The various devel-opmental stages of turbot eggs showed different sensitivities to alkaline waters, with the most sensitive stage being that of embryo segmentation, when a pH value of 8.0 killed half of the eggs (Volodin, 1960). Resistance increased after this stage, but even at pH 9.0, hatching was delayed. This was different from the results of our experiment, wherein eggs hatched in alkaline solutions, even at pH 10, with a hatching rate of about 24% (Fig. 3), followed by pH 9 (35%) and pH 8 (48%). These data suggested that catfish eggs were less sensitive to acid and alkaline stress compared to those of the above-mentioned fish species.
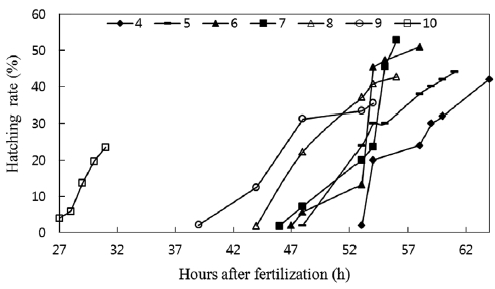
A significant difference was observed between hatching times, as shown in Fig. 4. Hatching time increased with de-creasing pH values. Although the hatching rate was lowest at pH 10, the hatching time was the shortest, with hatching be-ginning 27 h after fertilization and ending at the 31st h. The eggs in pH 4 solutions did not start hatching until 53 h after fertilization, and 64 h were required for all eggs to hatch. In this experiment, hatching time was longer than those recorded under field conditions, which may be attributable to experi-mental conditions being less stable than field conditions. The eggs in our experiment seemed to suffer more artificial stress from factors such as pH adjustment and water change. The delay in hatching time, as observed in catfish, has been fre-quently been noted in eggs of other fish species. Rask (1983) found that eggs of