Transgenic fish technology not only provides a unique model system for studying gene regulation in vertebrates but also offers a method to increase the efficiency of farming of many aquaculture-relevant species (Kapuscinski, 2005). In the last two decades, a number of fish species of diverse taxo-nomic groups have been subject to transgenic manipulation. Most transgenic studies on commercially important fish spe-cies have focused on growth enhancement by growth hormone (GH) transgenesis (Nam et al., 2007).
Many
Mud loach
>
Generation of GH construct driven by the lectin promoter
A mud loach genomic DNA library was screened with a probe, the DIG-labeled mud loach liver EST clone, which encoded mud loach hepatic C-type lectin. Multiple positive clones from the filter hybridization were analyzed by restric-tion mapping and shot-gun sequencing. Based on this se-quence analysis, the 5´-upstream sequence of the mud loach C-type lectin gene was characterized using several web-based bioinformatic tools. A 2.3-kb upstream fragment from the ATG start codon of the lectin gene was isolated by PCR using the primers mlectP-F (5´-AAGAGTGTGGCTTTGACCC-3´) and mlectP-R (5´-GGAAAAGTGACACATCTGC-3´), and a DNA template (500 ng) was prepared from the identified phage clone containing the mud loach lectin gene. PCR am-plification was carried out as follows: 30 cycles of 94℃ for 1 min, 60℃ for 30 s, and 72℃ for 1.5 min, with an initial denaturation step at 94℃ for 2 min. The PCR product was cloned into the pGEM-T Easy Vector (Promega, Madison, WI, USA), and recombinant clones with the correct insert size were sequenced in both directions. The lectin promoter was spliced from the pGEM-T Easy Vector plasmid using
>
Gamete collection and microinjection
Mature mud loach female and male brood fish were given an intraperitoneal injection of human chorionic gonadotropin at the doses of 10 IU and 2 IU per gram body weight, respec-tively (Kim et al., 1994). Eggs were artificially inseminated with sperm using the wet method, and the resultant embryos were held at 8℃ until used for microinjection. Microinjection was carried out into one-celled embryos with 100 μg/mL pm-lectGH in buffer (10 mM Tris-Cl, pH 8.0; 0.1 mM EDTA, pH 8.0; 0.05% phenol red) (Nam et al., 1999). After microinjec-tion, the embryos were transferred to a well-aerated incubator at 25 ± 1℃ until hatching. Hatching success and early surviv-al rate up to yolk sac absorption were estimated as percentages of eggs injected and larvae hatched, respectively, based on triplicate examinations using at least 110 embryos randomly chosen from each replicate batch. To prepare the positive con-trol for GH transgenesis, pmlβ-actGH, which had been previ-ously used for generating GH-transgenic mud loach (Nam et al., 2001), was microinjected into another batch of embryos, and the non-injected embryos were used as negative controls.
>
Body-weight frequency distribution, PCR typing, and founder growth trial
After hatching, 24 larvae from each injected batch were subjected to PCR amplification of the transgene to confirm successful transfer. DNA was prepared from whole body of larvae using a conventional sodium dodecyl sulfate/proteinase K method. PCR was carried out using a pair of prim-ers designed to amplify the junction fragment between the regulator and the structural GH gene for each transgene construct. Oligonucleotide primers were mlectP-1F (5´-GT-TATGGAGTCCCTCCCAA-3´) and mlGH-1R (5´-CAGC-CAGCTGGTGCAGGTG-3´) for the pmlectGH construct, and mlβ-actP-1F (5´-CCACGCGCTGAATCGGCGGC-3´) and mlGH-1R (see above) for the pmlβ-actGH transgene. Re-actions consisted of 30 cycles of 94℃ for 1 min, 58℃ for 1 min, and 72℃ for 1.5 min, with an initial denaturation step at 94℃ for 2 min. After confirmation of the successful transgene delivery to a portion of microinjected embryos, the remaining larvae in each group were reared at 25℃ in three separate tanks (50 L each for the pmlectGH-injected, pmlβ-actGH-injected, and non-injected groups) until 1 week of age. Fish were then transferred to one of three larger tanks (120 L) for 2 months’ further growth. Fish were fed a commercial carp diet on an ad libitum basis during the experimental period. At 2 months, the body weight (nearest 1 mg) of each individual was de-termined to evaluate the body-weight frequency distribution. The presumed transgenic founders with large body size were identified by PCR screening using the DNA template prepared from a caudal fin clip (Nam et al., 2003). PCR primers were as described above. Transgenic founder fish (PCR-positive for pmlectGH) were selected and grown to sexual maturity to be tested for germ-line transmission to the F1 generation.
>
Germ-line transmission and growth characteristics of F1 progeny
Seven founder pmlectGH-transgenic males were crossed with normal mud loach females to produce F1 progeny. Ar-tificial insemination was performed as described previously (Nam et al., 2001), and fertilization rate, hatching success, and early survival up to yolk sac absorption were examined as above. At least 48 randomly chosen larvae (3 days old) from each cross were screened by PCR amplification of the trans-gene, as described above, to determine the germ-line trans-mission frequency. At one month, fast-growing transgenic F1 individuals were again identified by PCR and subjected to a growth trial up to 6 months of age. Growth performances of transgenic progeny (
>
Viability of microinjected embryos and efficiency of gene transfer
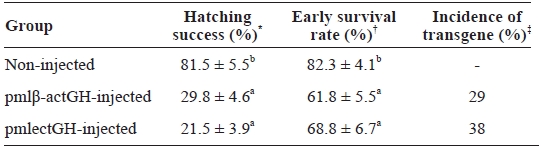
Non-injected control groups showed fairly good hatching success and early survival, whereas the microinjected groups revealed significantly reduced scores in both hatching success (29.8% for pmlβ-actGH and 21.5% for pmlectGH) and percent early survival up to yolk sac absorption (61.8 and 68.5% for pmlβ-actGH- and pmlectGH-injected groups, respectively) (Table 1). The lower viability of microinjected embryos was not surprising, as many previous studies have reported similar observations (Pandian and Marian, 1994; Gong et al., 2007). The adverse effects of microinjection on embryo viability are usually species specific. This harmful effect on viability arises from mechanical damage to the chorion and egg membrane during microinjection rather than from the exogenous DNA itself (Pandian and Marian, 1994). The incidence of transgene presence in 1-week-old fry from each microinjected group (38% for pmlectGH group and 29% for pmlβ-actGH) was higher than those previously reported (see Nam et al., 2007). However, the presence of extrachromosomal persistent trans-gene copies might also be, at least in part, responsible for such a high incidence. Exogenously introduced DNA usually un-dergoes formation of multiple concatemers, which frequently persist not only in early embryos but also in adults for a long period (Nam et al., 1999; Kim et al., 2004; Gong et al., 2007).
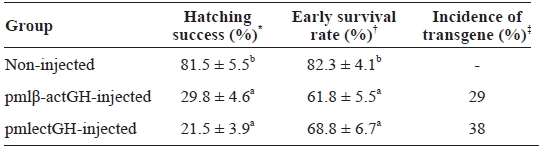
Hatching success early survival up to yolk sac absorption and incidence of transgene in microinjected and non-injected control groups of mud loach Misgurnus mizolepis
>
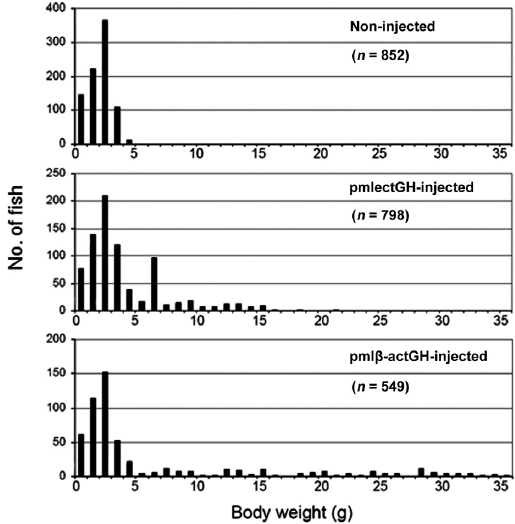
Body-weight frequency distribution
The body-weight frequency in non-injected controls at 2 months showed a normal distribution with a mean of 2.6 g (Fig. 1). The group injected with pmlβ-actGH also displayed a normal distribution, but many individuals deviated from this, as expected (see also Nam et al., 2001). Of the 549 individu-als that developed from embryos injected with pmlβ-actGH construct, 138 presumed transgenic founders exhibited signifi-cantly heavier body weights than those of individuals belong-ing to the largest class in the non-injected control group. More importantly, a number of pmlβ-actGH transgenics showed extraordinarily heavy weights, more than 10 times the aver-age body weight of the control group; the largest transgenic individuals reached 36 g. Meanwhile, many individuals in the pmlectGH-injected group (
PCR screening suggested that most, but not all, such large individuals contained the corresponding transgene constructs in their fin tissues (PCR gels not shown). However, several individuals were unexpectedly PCR negative despite their ex-traordinarily large body sizes. This might be explained by a mosaic transgene distribution across tissues, as we tested only fin tissue (Rahman and Maclean, 1999; Dunham, 2004). Al-though constructs were introduced into one-celled embryos, transgenes may have been integrated into host chromosomes after several rounds of cell division, leading to an extremely high level of mosaicism in these individuals. The late integra-tion of transgenes into fish genomes has often been observed, especially when the DNA was introduced by microinjection (Iyengar et al., 1996). Further examination based on PCR as-says of diverse tissues is needed to determine whether such individuals are true transgenic founders harboring transgene constructs. In spite of these questionable individuals, it was evident that the early growth responses of transgenic mud loaches to both GH constructs differed substantially. The ef-fect of pmlectGH transgenesis on growth stimulation in trans-genic mud loach was more moderate than that of pmlβ-actGH transgenesis.
>
Germ-line transmission frequency
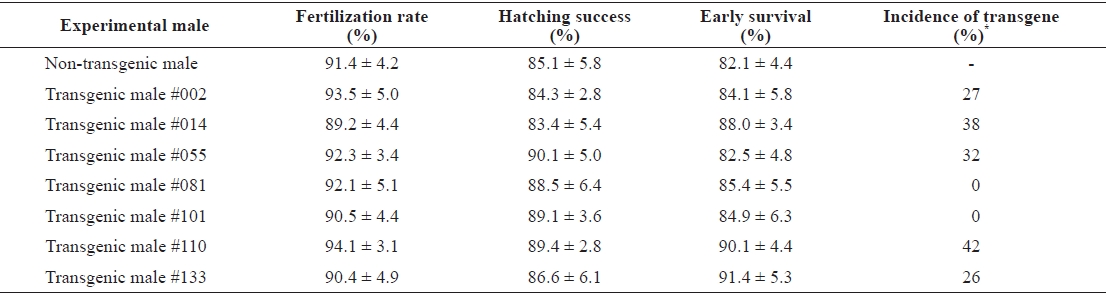
Of seven putative transgenic male founders, only five transmitted their transgene construct to the next generation. Fertilization rates, hatching success, and survival up to yolk sac absorption were similar in all families, including the non-transgenic control cross (
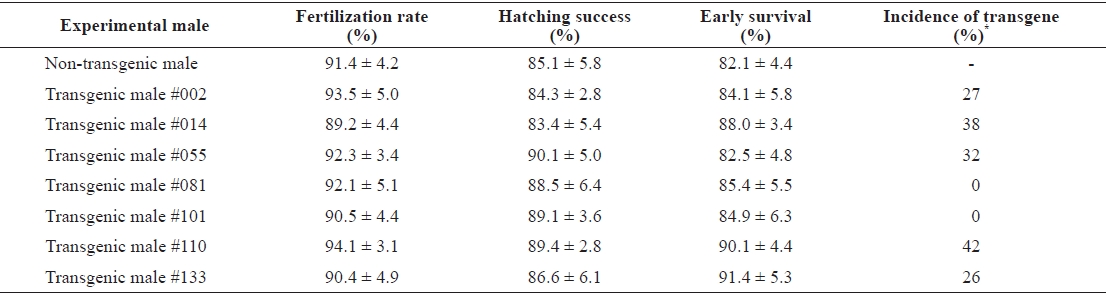
Germ-line transmission of pmlectGH transgene from founder transgenic males to F1 progeny of mud loach Misgurnus mizolepis
jection of meganuclease (
>
Growth characteristics of F1 progeny
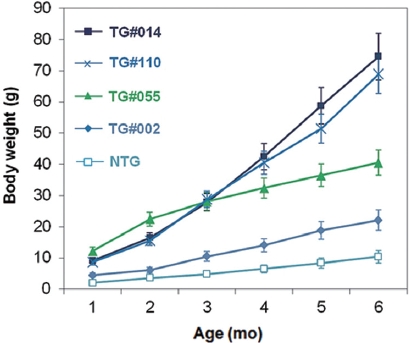
Growth stimulation of pmlectGH transgenics was also re-producible in the F1 generation. Transgenic F1 lines showed strain-dependent growth stimulation, ranging from two- to seven-fold increases in body weight relative to that of non-transgenic siblings when assessed at 6 months of age (Fig. 2). Strain-specific growth patterns were also found. For example, transgenic line TG#055 showed the fastest early growth, but later stabilized. In contrast, TG#014 showed a consistent slope of the growth curve until the end of the growth trial. Trans-gene-based growth stimulation in fish is governed by a num-ber of factors associated with transgenic status, such as trans-gene copy number, transgene integration site, and the fashion of GH expression (Rahman et al., 2000; Devlin et al., 2004), which will therefore be key subjects in future studies with these transgenic lines. Although we have not yet quantitated GH transcript and protein levels, it is generally agreed that the lectin regulator is weaker than the previously used β-actin promoter in terms of moderate growth stimulation across two generations. Unlike pmlβ-actGH transgenics that exhibited a greater than 35-fold growth acceleration (Nam et al., 2001, 2007), GH transgenesis driven by the lectin regulator resulted in maximum six- to seven-fold growth stimulation, even for the transgenic hemizygous genotype. Furthermore, the severe abnormalities caused by excess GH observed in several pmlβ-actGH transgenics was not noted in transgenic individuals harboring the pmlectGH construct. Furthermore, gigantism, a phenomenon typical of pmlβ-actGH transgenics, was di-minished in pmlectGH transgenesis (Nam et al., 2001); the body size of most fast-growing pmlectGH transgenics was not greater than the normal body size of this species (maximum ~100 g under laboratory conditions).
In summary, GH transgenesis using a lectin regulator in an autotransgenic manner was performed in mud loach and resulted in growth improvement and germ-line transmission. The growth characteristics of pmlectGH transgenics were different from the pattern observed in the pmlβ-actGH con-struct: growth stimulation of pmlectGH transgenics was more moderate and stable; no severe abnormalities or extraordinary gigantism occurred. These data thus provide a good basis for the practical application of GH-transgenic mud loaches to aquaculture.