The objectives of this research were to improve the film-forming properties of Channel Catfish Ictalurus punctatus skin gelatin (CSG) by cross-linking with transglutaminase (TG), determine and optimize the TG reaction time, and characterize the mechanical and barrier properties of CSG edible film. Cross-linking of CSG was performed by TG for 0, 10, 20, 30, and 40 min at 50℃, and the reaction was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The color and mechani-cal and barrier properties of edible films fabricated with CSG cross-linked with TG were characterized. Gelatin yields from the extraction ranged from 18.2% to 23.3%. SDS-PAGE exhibited dark bands at 120 and 250 kDa, indicating successful TG-mediated cross-linking. The color of CSG film was not affected by TG cross-linking. The tensile strength of CSG films cross-linked with TG decreased from 42.59 to 21.73 MPa and the percent elongation increased from 42.92% to 76.96% as reaction times increased from 0 to 40 min. There was no significant difference in water vapor permeability of CSG films.
Gelatin is an important biopolymer and widely used to manufacture gel desserts, edible films in the food industry, and hard and soft capsules in the pharmaceutical industry (Choi and Regenstein, 2000). The principal conventional sources of gelatin are mammalian pig skins and cow hides; however, for a number of reasons, such as religious prohibitions under Judaism and Islam and diseases such as bovine spongiform encephalopathy, alternative sources of gelatin are now in de-mand (Leuenberger, 1991; Choi and Regenstein, 2000; Mon-tero and Gomez-Guillen, 2000; Sarabia et al., 2000).
Chemical properties of mammalian and fish gelatins differ. Fish gelatins have low gelling and melting temperatures, but relatively high viscosities (Leuenberger, 1991). Researchers have investigated several approaches to alter fish gelatin in an effort to enhance its functionality. Blending of fish gelatin with biopolymers, in conjunction with plasticizers, is one way to improve its properties (Chen et al., 2003; Uresti et al., 2003; Haug et al., 2004). Furthermore, the addition of plasticizers such as glycerol, sorbitol, sucrose, and polyethylene glycol can improve the mechanical properties of fish gelatin films or gels (Tanaka et al., 2001; Vanin et al., 2005). The addition of chemical cross-linking enzymes such as microbial transglu-taminase (TG) has also been proven to enhance the proper-ties of fish gelatin (Tseng et al., 1990). Extensive research has focused on the basic characteristics and applications of fish gelatin gels treated with microbial TG (Ramirez et al., 2000; Fernandez-Diaz et al., 2001; Uresti et al., 2003; Kolodzlejska et al., 2004).
Farm-raised catfish are important to the economy of small fish farms, and about 55% of the product of catfish process-ing is byproduct that is sold for a low price (Prinyawiwatkul et al., 2002). Therefore, the utilization of catfish byproduct is of great interest and could be beneficial to the catfish farming industry. Therefore, if it is possible to make fabricate gela-tin edible films from the gelatin extraction of catfish skins, fish gelatins could be substituted for mammalian gelatins. Although some fish gelatins are currently available commer-cially, they are not well-characterized. Most studies on gelatin refer to mammalian gelatins, and few studies to fish gelatins have been performed (Leuenberger, 1991; Choi and Regen-stein, 2000; Gilsenan and Ross-Murphy, 2000; Montero and Gomez-Guillen, 2000; Sarabia et al., 2000). Therefore, the objectives of this research were to improve the film-forming properties of channel catfish skin gelatin (CSG) extract by cross-linking with TG, determine and optimize the TG reac-tion time in CSG solution, and characterize the mechanical and barrier properties of CSG edible film.
>
Cleaning catfish skins and extraction of CSG
Frozen catfish skins were obtained from Harvest Select Incorporated (Uniontown, AL, USA). Catfish skins were trimmed to remove residual fish meat and fin, cut into small pieces (approximately 20 × 20 mm2), and washed with 4℃ deionized (DI) water three times. The cleaned fish skins were drained and squeezed by cheesecloth for 5 min to remove ex-cess water (Zhou and Regenstein, 2004).
Pretreatment procedures were followed by methods previ-ously developed by Zhang et al. (2007). Catfish skin (40 g) was treated with 240 mL 0.25 M sodium hydroxide (NaOH) at 4℃ for 1 h, and then washed with 4℃ DI water, followed by treatment with 240 mL 0.09 M acetic acid at 4℃ for 1 h. The CSG was extracted with 120 mL DI water at 50℃ in a water bath (Isotemp 2L; Thermo Fisher Scientific Inc., Waltham, MA, USA) for 3 h. The mixtures were filtered through glass wool and freeze-dried.
Protein concentrations in CSG extract were determined by the Biuret method by bovine serum albumin as a standard. The gelatin yield, expressed as protein yield, was calculated as follows (Zhou and Regenstein, 2004):
Protein yield (%) = (protein concentration [g/mL] × volume of extract [mL/weight of catfish skin (g)]) × 100.
>
Preparation of enzymatic cross-linked CSG solution
CSG solution was prepared by mixing 18 g gelatin (3% gel-atin) and 5.4 g glycerol (30% glycerol based on gelatin con-tent) in 600 mL DI water. The solute was dissolved at 50℃ for 1 h. After dissolving, 0.1 M NaOH was added until the pH reached approximately 7.0. The solution was then centrifuged at 5,000 g for 10 min at 25℃. After centrifugation, the solution was kept in a water bath (Isotemp 2L; Thermo Fisher Scientific Inc.) at 50℃ until use.
TG (Ajinomoto ACTIVA T1) was purchased from Ajino-moto Food Ingredients LLC (Paramus, NJ, USA). The en-zyme consisted of 99% lactose and 1% TG according to the manufacturer’s specifications. TG powder (1,200 mg) was dissolved in 15 mL DI water and mixed by a Touch Mixer (Vortex Mixer; Thermo Fisher Scientific Inc.) The TG solution was centrifuged at 5,000 g for 15 min at 0℃ by a Sorvall RC 5B Plus centrifuge (Kendro Laboratory Products, Asheville, NC, USA). After centrifugation, the supernatant was removed. The pellets were obtained and added to 15 mL DI water. The TG solution (1,200 mg in 15 mL water) was then added to the gelatin solution. This was then aliquoted into 5 beakers, which corresponded to reaction times of 0, 10, 20, 30, and 40 min in a water bath (Isotemp 2L; Thermo Fisher Scientific Inc.) at 50℃. TG deactivation was accomplished by boiling for 5 min.
>
Fabrication of CSG film cross-linked with TG
For the film-forming solutions, 1% gelatin and 20% glycer-ol contents were selected based on previous reports (Sobral et al., 2001; Jongjareonrak et al., 2006). After TG deactivation, TG-treated gelatin solutions were poured onto a BYTAC type VF-81 FEP (SPI Supplies/Structure Probe, Inc., West Chester, PA, USA) coated glass plate (25 × 25 cm2). The plates were balanced before adding the gelatin mixture to ensure even dis-tribution of film solution. During pouring, the solutions were filtered through 2 layers of cheesecloth to remove impurities and bubbles. The poured solutions were dried at room tem-perature (approximately 25℃) for 24 h. The film was checked after 12 h to ensure that conditions were favorable for the pro-duction of a good film. Dried films were then carefully re-moved from the plates (Zhang et al., 2007).
>
Sodium dodecyl sulfate-polyacrylamide gel elec-trophoresis
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed with a Ready Gel Precast Gel System (Bio-Rad Laboratories, Inc., Hercules, CA, USA) to determine the molecular weight (MW) profile of the CSG extracts (Laemmli, 1970). A pre-cast 4% stacking gel and 10-20% continuous gradient separating gels (Sigma-Aldrich Chemical Co., St. Louis, MO, USA) were used. After measur-ing the protein concentrations, the gelatin extracts were dilut-ed to approximately 1.0 mg/mL with 62.5 mM Tris buffer and boiled for 3 min in a water bath (Isotemp 2L; Thermo Fisher Scientific Inc.). An aliquot (15 μL) from each CSG diluent was loaded onto the stacking gel. The PageRuler Prestained Protein Ladder Plus molecular weight marker mix (Fermen-tas, Hanover, MD, USA) with MW from 10 to 250 kDa was used as a standard. An electrical field of 0.4 volt/mm was ap-plied to the gel, and increased to 0.75 volt/mm once the dye fronts reached the separating gel (Zhang et al., 2007).
>
Determination of gelatin film color
The Hunter color scale of lightness (L), redness (a), and yellowness (b) of catfish films was determined by a CR-300 Minolta Chromameter (Minolta Camera Co., Osaka, Japan). The light source was illuminant C, and the white plate (CR-A43; L = 96.86, a = -0.02, and b = 1.99) provided by the man-ufacturer was used for calibration and background.
Tensile strength (TS) and percent elongation (%E) of CSG films were determined by following the standard procedures of ASTM D 882-97 (American Society for Testing and Ma-terials 1998) by a Mini-44 Instron (Instron Co., Canton, MA, USA). The CSG films conditioned in an environmental cham-ber (Model 435314; Hotpack Corp., Philadelphia, PA, USA) at 25℃ and 50% relative humidity for 16 h were cut into 20 × 50 mm2 strips, and the thickness of each strip at three locations (top, center, and bottom) was measured and recorded by a hand-held electronic digital micrometer (CO030150F6; Mara-thon Watch Company, Ltd., Richmond Hill, ON, Canada). The mean of the three thickness measurements of each strip was used to calculate TS and %E of the film. Each strip was fas-tened onto screw-action grips with an initial 20 mm gap (the distance between the top and bottom grip), and the strip was stretched using the grips at a head speed of 50 mm/min until broken. The TS of CSG films was calculated as follows:
TS = peak load (N)/initial cross-sectional area (m2).
%E was calculated as follows: %E = final gap (mm)/initial gap (mm) × 100.
Water vapor permeability (WVP) was determined follow-ing the ASTM E96-95 standard method (American Society for Testing and Materials 1999) using Fisher permeability cups (Thermo Fisher Scientific Inc.). The CSG films were cut into 55-mm-diameter circles, and their thickness was measured at five locations in the circle (top, bottom, left, and right 10 mm from the edge and center). The mean film thickness was used to calculate WVP. Each circle was mounted on the test cell and tightly fastened with the top cover. A thin layer of silicone high-vacuum grease (Dow Corning, Midland, MI, USA) was used as a sealant to avoid vapor leaks through cup joints. Af-ter measuring the initial weight, the test cups were placed in an environmental chamber (Model 435314; Hotpack Corp.) at 25℃ and 50% relative humidity for 24 h, and weight changes were recorded. The WVP of the films was calculated as fol-lows:
WVP (g/Pa/s/m) = water permeance (g/Pa/s/m2) × thickness (m),
where water permeance = WVT/ΔP, WVT = weight change/(time [h] × area [m2]), and ΔP = vapor pressure differences across the film (mm Hg).
Each experiment was repeated six times. One way analysis of variance (ANOVA) was performed using the SPSS version 11.5 (SPSS Inc., Chicago, IL, USA). The differences among means were analyzed using least significant difference, and the significance level was defined as
The gelatin yields ex-pressed as protein yield from the extraction method ranged from 18.2% to 23.3%. The gelatin yield extracted from Alaskan pollock skin using a pretreat-ment method similar to ours was approximately 18% (Zhou and Regenstein, 2004). Gudmundsson and Hafsteinsson (1997) also obtained gelatin yields of 11-14% from cod skin using a similar pretreatment method.
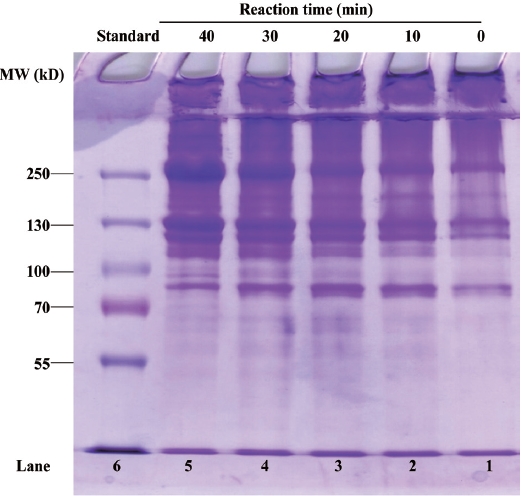
The molecular mass profile of the gelatin films produced in the presence of TG (Fig. 1). The band profiles of SDS-PAGE at 0 min exhibited a typical CSG band profile, two darker bands at ~120 and ~250 kDa (Lane 1) (Zhang et al., 2007). According to Zhou and Regenstein (2004), the 250 kDa band is the β-chain of collagen and that at 120 kDa is the α-chain. The introduction of TG to the film solutions resulted in the formation of high molecular mass polymers, exhibiting a much darker band at 250 kDa (lanes 2-5). As the TG reac-tion time increased from 10 to 40 min, more collagen β-chain was formed by TG. These results indicate that TG cross-linked the fish gelatin matrix, forming higher-molecular-weight fish gelatins.
Similar results were reported for megrim skin and mammal gelatins (Lim et al., 1999; Gomez-Guillen and Montero, 2001; Yi et al., 2006). The authors observed decreasing band intensi-ty at ~100 kDa and increasing band intensity in the upper part of the gel when the gelatin was treated with TG. Ramirez et al. (2002) reported the same in fish proteins, such as myosin, due to TG-induced cross-linking.
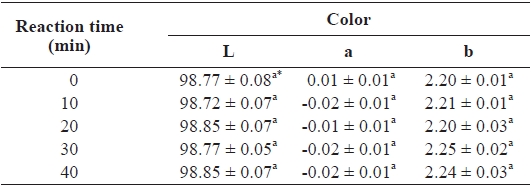
The lightness (L), redness (a), and yellowness (b) of the CSG films were not significantly affected by either TG cross-linking or reaction time (Table 1). Yi et al. (2006) reported that the L of films cross-linked with TG was not significantly affected by reaction time, in agreement with our observation. However, they observed that the red/green (a) and yellow/blue (b) values varied from -0.19 ± 0.01 to -0.23 ± 0.00 and 3.36 ± 0.06 to 3.47 ± 0.09, respectively, indicating that films be-came more greenish with time. No significant difference in the color of catfish films cross-linked with TG was observed in this study, presumably due to our use of a different type of fish gelatin.
Effect of reaction time of transglutaminase on color of channel catfish Ictalurus punctatus gelatin films
>
Mechanical properties of CSG film cross-linked with TG
CSG films cross-linked with TG ranged from 0.046 ± 0.0009 to 0.0493 ± 0.0018 mm thick. These values were used to calculate the TS and %E of the films. In general, gelatin films possess greater TS than that of other protein films, such as casein or whey films (Chambi and Grosso, 2006). This was related to the organizational level of the protein network. Gel-atin possesses a relatively disorganized structure, but it can re-nature during the gelling and film-forming process, partially reacquiring the collagen triple helix structure, a protein with a high degree of organization (Achet and He, 1995). According to Siew et al. (1999), the increase in chain organization likely optimizes molecular packing, resulting in films with desirable mechanical and barrier properties.
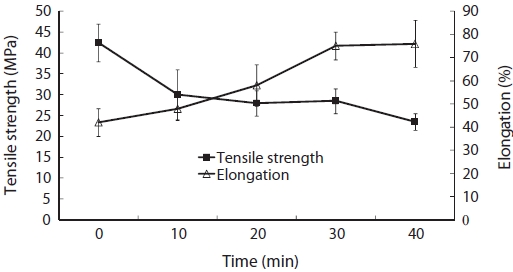
A decrease in TS and simultaneous increase in %E is a characteristic property of other protein or polysaccharide films plasticized with different hydrophilic compounds (Arvanitoy-annis and Biliaderis, 1998; Tanaka et al., 2001). The introduc-tion of plasticizers into the polymeric matrix also results in higher %E values. Plasticizers decrease intermolecular attrac-tive forces and increase biopolymer chain mobility; as a result, film flexibility and extensibility can be improved (Gennadios and Weller, 1990). The CSG films that cross-linked with TG and which contained 20% glycerol as a plasticizer exhibited lower TS and higher %E values (Park et al., 2008).
The effects of TG cross-linking on the mechanical proper-ties of gelatin films remain to be resolved. With respect to TG treatment, the introduction of cross-linkages did not result in significant changes in TS compared to untreated films (Cham-bi and Grosso, 2006). Oh et al. (2004) and de Carvalho and Grosso (2004) obtained similar results for milk protein and type B gelatin films cross-linked with TG. Babin and Dick-inson (2001) observed that treatment with TG could result in both positive and negative results with respect to the strength of gelatin films. Also, Yi et al. (2006) reported that the TS of cross-linked fish gelatin films increased from 48.03 to 68.00 MPa and that the %E decreased from 13.1 to 1.47%, as reac-tion time increased from 0 to 50 min.
However, our data suggest that the TS of CSG films cross-linked with TG decreased from 42.59 to 21.73 MPa and that the %E increased from 42.92% to 76.96% as the reaction time increased from 0 to 40 min (Fig. 2). These results are in good agreement with those of other investigators. Lim et al. (1999) reported that the TS of gelatin films were comparable to those of other protein films. Although direct comparison between samples was unreliable due to varying test conditions and sub-strates, the TG cross-linked gelatin films exhibited much high-er %E values (Lim et al., 1999). Films produced by TG had higher %E values than those that did not use TG (Chambi and Grosso, 2006). Daniels (1989) speculated that cross-linking of gelatin by TG could increase %E because the structure of the overall materials shifts from that of individual chains associ-
ated with many weak van der Waals forces, to chains bound by a few, but strong, covalent bonds.
>
WVP of CSG film cross-linked with TG
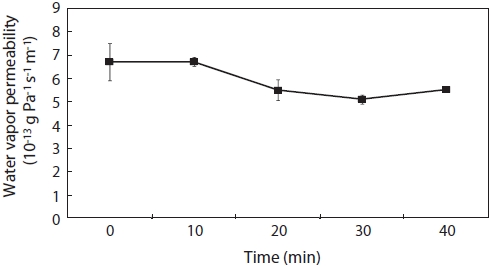
The WVP values of CSG films ranged from 5.19 to 6.82 × 10-13 g Pa-1 s-1 m-1 (Fig. 3). WVP exhibited no significant differences as TG reaction time increased. These results are in agreement with other reports. Although de Carvalho and Grosso (2004) reported that the WVP coefficient was lower in bovine gelatin films cross-linked with TG than in an untreated gelatin film, Piotrowska et al. (2008) observed that the water barrier properties of fish skin gelatin were not altered by TG cross-linking.
Addition of glycerol to film solutions usually worsens the water barrier properties of polysaccharide or protein films (Butler et al., 1996; Arvanitoyannis et al., 1998). Here we used glycerol, which may explain the low WVP values compared to other films. Yi et al. (2006) observed that WVP was ont affected by TG reaction time, which may have been due to the effect of the plasticizer. The authors found that addition of a hydrophilic plasticizer, favoring the adsorp-tion and desorption of water molecules, improved the WVP of hydrocolloid-based films. The film to which 20% D-sorbitol was added as a plasticizer exhibited an increased WVP due to its hydrophilic activity, thus masking the effect of TG-induced cross-linking.
In conclusion, upon TG cross-linking, the molecular mass profile of gelatin films increased with reaction time. The TG cross-linking reaction did not affect the color of the films. However, cross-linking resulted in a significant decrease in TS, and a significant increase in %E, with increasing reaction time. Therefore, cross-linking of CSG with TG may improve the flexibility of gelatin films without affecting its barrier properties. Further research on the potential application of TG to fish gelatin films in the food and pharmaceutical industries is now necessary.