The matrix metalloproteinases (MMPs) are a family of zinc-dependent endopeptidases that play important roles in the degradation of extracellular matrix (ECM), and in a variety of biological and pathological processes, such as tumor invasion and metastasis (Sternlicht and Werb, 2001; Nishida et al., 2008; Hwang et al., 2010; Khan et al., 2010). This family of proteinases has includes at least 28 endopeptidases collectively capable of degrading virtually all ECM components. MMPs family members have been classified into eight subgroups according to their structural and functional characteristics. These are the minimal-domain MMPs, simple hemopexin domain containing MMPs, gelatine-binding MMPs, furin activated secreted MMPs, and vitronectin-like insert MMPs, and membrane bound MMPs include type I trans-membrane MMPs, glycosyl-phosphatidyl inositol-linked MMPs, and type II transmembrane MMPs (Egeblad and Werb, 2002). MMPs regulate the synthesis and secretion of cytokines, growth factors, hormone receptors and cell adhesion molecules. They also contribute to cell growth and development, cell morphogenesis, tissue remodelling, angiogenesis, cardiovascular, allergies, neurodegenerative diseases, some cancers, and a series of physiological and pathological processes (Sang et al., 2006; Hu et al., 2007; Kong et al., 2008).
Gelatinases (MMP-2, MMP-9) play an important role in cancer invasion and metastasis, and have been detected most consistently in malignant tissues. This subgroup of metal-loproteinases has also been called type IV collagenases, because of their ability to cleave type IV collagen (Salo et al., 1985). One of them, MMP-2 (gelatinase-A, 72 kDa type IV collagenase), was originally purified from a highly progressing metastatic murine tumor (Wilhelm et al., 1989). MMP-2 binds to type I collagen through the fibronectin domain, which stabilizes it against autolysis, thereby controlling its activity (Ellerbroek et al., 2001). MMP-2 expression is dependent on extracellular matrix metalloproteinase inducer, growth factors, cytokines, and hormones. Pro-MMP-2 activation needs contributions from MT1-MMP and TIMP metallopeptidase inhibitor 2. A low level has been linked to a favorable prognosis in patients with hormone receptor-negative tumors, usually associated with poor prognosis. As a zymogen that requires proteolytic activation for catalytic activity, MMP-2 has been implicated in invasion and metastasis in many cancer model systems, including human breast cancer (Jezierska and Motyl, 2009; Fredrich and Illing, 2010). MMP-9 (92 kDa type IV collagenase, gelatinase B) is produced in human macrophages and polymorphonuclear leukocytes. It has also been localized in endothelial cells and synovial fibroblasts in rheumatoid arthritis synovium. MMP-9 is expressed by osteoclasts in human normal bone tissues, indicating a role in bone remodelling. Intact mature human odontoblasts also express MMP-9, and it has been detected in human dental caries lesions and saliva. However, MMP-9 is not expressed by human gingival fibroblasts. Like MMP-2, MMP-9 may exist in the ECM bound to type I collagen, gelatine, or laminin (Bolcato-Bellemin et al., 2000).
Degradation of denatured collagen I by MMP-2 and MMP- 9 is readily demonstrable in biological materials. One technique, known as gelatin zymography, identifies gelatinolytic activity in biological samples using sodium dodecyl sulfate (SDS)-polyacrylamide gels impregnated with gelatine. The human fibrosarcoma cell line HT1080 has been used extensively in studies of ECM proteins involved in attachment, invasion, and metastasis. Additionally, HT1080 cells are used because they produce both MMP-2 and MMP-9 enzymes (Brooks and Schumacher, 2001).
The abalone
In the present study, to investigate the bioactive potential of abalone intestine, abalone intestine G I digests (AIGIDs) were separated into various molecular weight (MW) fractions by ultrafiltration. We focuses on AIGID II and III mediated inhibition of both MMP-2 and MMP-9 and cancer cell migration in human fibrosarcoma HT1080 cells.
Live adult abalones were collected from Wando Island, Korea. Intestinal organs (guts) were separated from the washed abalone and lyophilized. Dulbecco’s modified Eagle’s medium (DMEM), trypsin-ethylenediaminetetraacetic acid (EDTA), penicillin/streptomycin, and fetal bovine serum (FBS) were obtained from Gibco BRL, Life Technologies (Grand Island, NY, USA). HT1080 cells were obtained from the American Type Culture Collection (Manassas, VA, USA). Primary and secondary antibodies, including MMP-2 (sc-13595), MMP-9 (sc-10737), NF-κB p65 (sc-8008), NF-κB p50 (sc-166588), β-actin (sc-130656), goat anti-rabbit IgG-horseradish peroxidase (HRP) (sc-2004) and goat anti-mouse IgG1-HRP (sc- 2060), were purchased from Santa Cruz Biotechnology-Inc (Santa Cruz, CA, USA). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), gelatin (type A) and phorbol 12-myristate 13-acetate (PMA) were from Sigma Chemical Co. (St. Louis, MO, USA). Other chemicals and reagents used were of analytical grade.
>
Preparation of AIGIDs using ultrafiltration (UF) membrane bioreactor
The method of Kapsokefalou and Miller (1991) was used to prepare AIGIDs (AIGID and AIGID I-III). Abalone intestine solution (1 L) was adjusted to pH 2.2 for gastric digestion using 1 M HCl and 10 M NaOH. Pepsin (EC 3.4.23.1) was added at an enzyme:substrate ratio of 1:100 (w/w), then incubated at 37℃ on a shaker for 2 h. The pH was adjusted to 6.5 to match the conditions of small intestinal digestion. Trypsin (EC 3.4.21.4) and α-chymotrypsin (EC 3.4.21.1) were added at an enzyme to substrate ratio of 1/100 (w/w), then incubated at 37℃ for 2.5 h. AIGIDs were centrifuged (10,000 g, 15 min, at 4℃), and the supernatant was lyophilized to obtain AIGID powder. AIGID (100 g) was solublized in 2 L of water and fractionated using UF membranes with a 100 kDa MW cut-off (MWCO). The filtrate was then fractionated further through a 10 kDa MWCO filter. All samples (AIGID and AIGID I-III) were dialyzed through a 1 kDa membrane and lyophilized.
Human fibrosarcoma HT1080 cells were cultured in DMEM containing 10% FBS and antibiotics as monolayers in 10 cm culture dishes at 37℃ in a humidified 5% CO2 atmosphere.
Cytotoxic effects of the AIGIDs on cultured cells were measured using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. To determine the cytocompatible effects of AIGIDs, HT1080 cells were seeded in 96-well plates at a 1×104 /well and incubated with various AIGID, AIGID I, AIGID II, and AIGID III concentrations. After a 24 h incubation, MTT reagent (50 μL) was added to each well and incubation was continued for another 4 h. Finally, DMSO was added to solubilize the formazan salt formed and the absorbance at 540 nm was determined using a microplate reader. Cell viability relative to the non-treated group was calculated. Data are expressed as means of at least three independent experiments and
The gelatinase activities of MMP-2 and MMP-9 were determined by gelatin zymography (Ta
>
In vitro wound migration assay
HT1080 cells were seeded in six-well plates. Cells were pretreated with mitomycin C (25 μg/mL) for 30 min before an injury line was made with a 2 mm wide tip on cells that were plated in culture dishes at 80% confluence. Cells were then washed with phosphate buffered saline. The cells were allowed to migrate in serum-free medium in the presence of various AIGID II and AIGID III concentrations for 24 h. Results were observed by microscopy.
Cultured cells were harvested and then lysed with RIPA buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 2 mM EDTA, and 0.1% SDS). Protein concentration was determined by the BCA method. Proteins were separated by 10% SDS-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. Membranes were blocked with 5% skim milk in TBS-T buffer (20 mM Tris-HCl, pH 7.6, 136 mM NaCl, 0.1% Tween-20) and then incubated with primary antibody (1:500
dilution) in blocking agent at 4℃ overnight. After washing with TBS-T, the membrane was incubated with secondary antibody (1:5,000 dilution) for 2 h at room temperature. Bands were developed by enhanced chemiluminescence and visualized with LAS-4000 imaging system (Fujifilm, Tokyo, Japan). Basal levels of MMP-2 and MMP-9 protein expression were normalized by assessing the level of β-actin protein.
Freeze-dired AIGID II and AIGID III (20 mg) was hydrolyzed with 6 M HCl at 110℃ for 24 h in vacuum. Amino acids derived with phenylisothiocyanate were identified and quantified using an automatic amino acid analyzer (Biochrom 20; Pharmacia Biotech, Cambridge, UK).
>
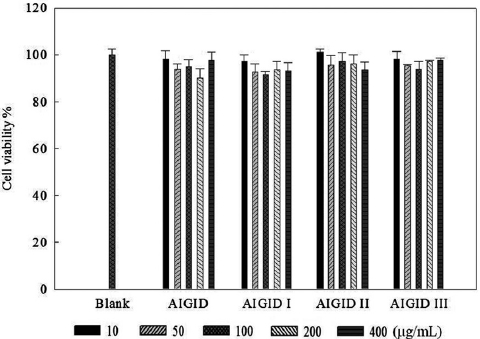
Cytocompatible effects of AIGIDs in HT1080 cells
The effects of AIGID and AIGID I-III on proliferation of HT1080 cells were evaluated using an MTT assay. No AIGID had any cytotoxic effect on HT1080 cells up to 400 μg/mL (Fig. 1).
>
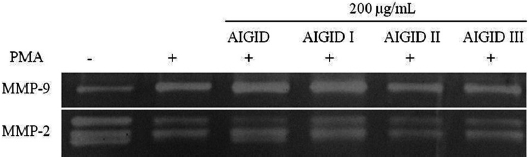
AIGIDs inhibit MMP-2 and MMP-9 activity in HT1080 cells
The effects of AIGIDs of differing MWs on MMP-2 and MMP-9 were evaluated. Cells treated with AIGID, AIGID I, AIGID II, or AIGID III at 200 μg/mL were stimulated with PMA. Conditioned media was used to assess MMP-2 and -9 activities. AIGID II and AIGID III showed marked inhibitory effects on MMP-2 and MMP-9 in HT1080 cells compared with the PMA treatment group (Fig. 2). However, neither AIGID nor AIGID I significantly inhibited MMP-2 or MMP-9. Thus, AIGID II and III were used in subsequent experiments.
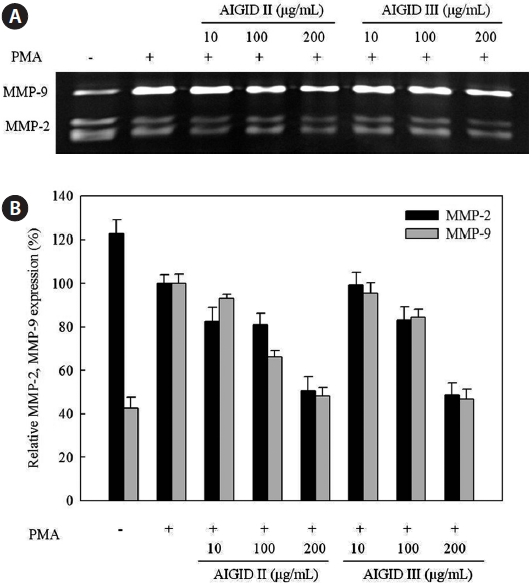
A gelatin zymography assay was performed to assess the dose-dependent inhibitory activities of AIGID II and III on MMP-2 and MMP-9 mRNA expression in HT1080 cells. MMP-2 and MMP-9 expression was suppresed by AIGID II and III in a dose-dependent manner (Fig.3). AIGID III showed greater inhibition of MMP-2 and MMP-9 expression than did AIGID II at 200 μg/mL.
>
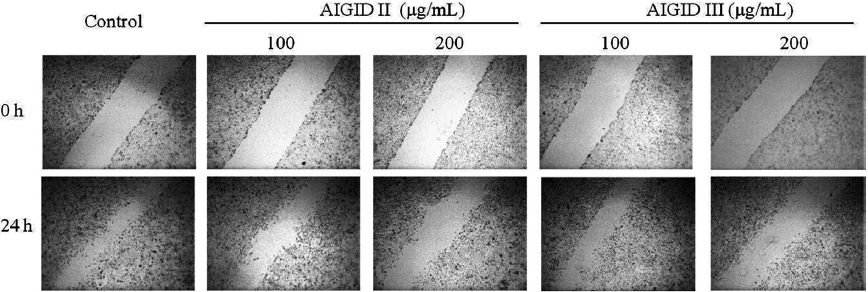
In vitro effects of AIGID II and III on migration of HT1080 cells
Cell migration is required for cancer cell invasion through the basement membrane. HT 1080 cells were treated with 0-200 μg/mL AIGID II and III. Both AIGID II and III inhibited the migration of HT1080 cells at 200 μg/mL (Fig. 4).
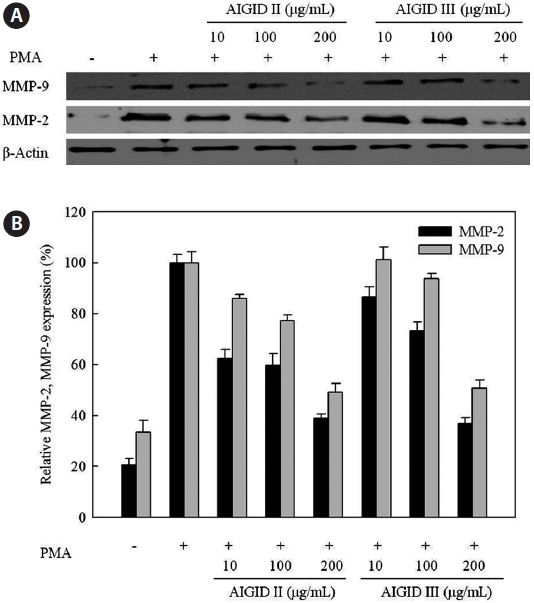
AIGID II and III mediated inhibition of MMP-2 and MMP- 9 protein levels were analyzed by Western blotting. AIGID II and III (10, 100, 200 μg/mL) suppressed MMP-2 and MMP-9 protein levels in a concentration-dependent manner, compared with PMA treatment (Fig. 5). Inhibition of MMP-2 expression by both AIGID II and III (200 μg/mL) was more noticeable.
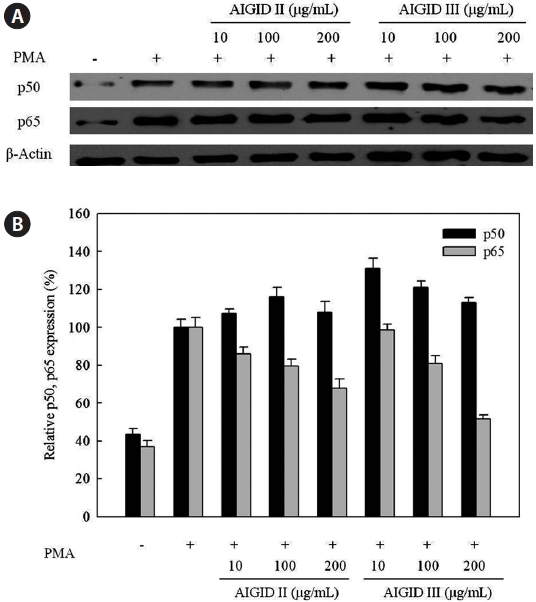
Furthermore, Western blots were used to assess the downregulation of protein expression and to determine the possible mechanisms for the effects of AIGID II and III on the expression of activator NF-κB. Western blotting was conducted to confirm down-regulation of p50 and p65 protein expression; both are components of NF-кB. AIGID II and III treatment resulted in decreased p65 protein level; this was dose-dependent (10, 100, 200 μg/mL) (Fig. 6). Additionally, AIGID III showed a greater inhibition of p65 expression than AIGID II at 200 μg/mL. However, no significant inhibition of p50 expression was detected at the concentrations tested. These results demonstrate that AIGID II and III down-regulated MMP-2 and MMP-9 expression through transcriptional down-regulation of NF-κB.
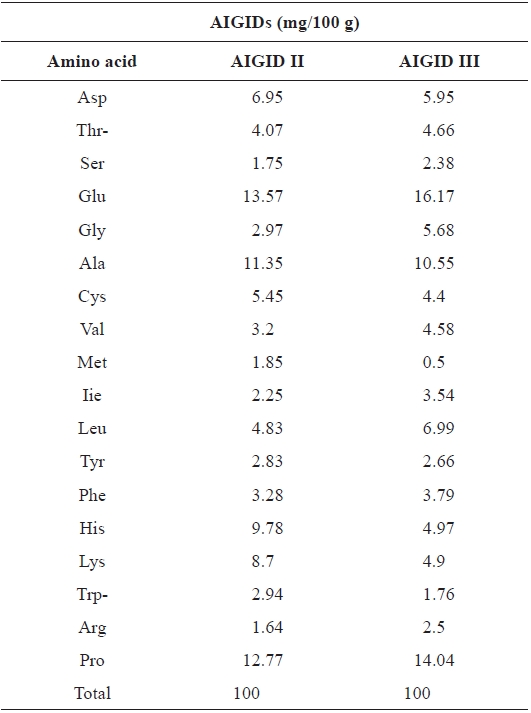
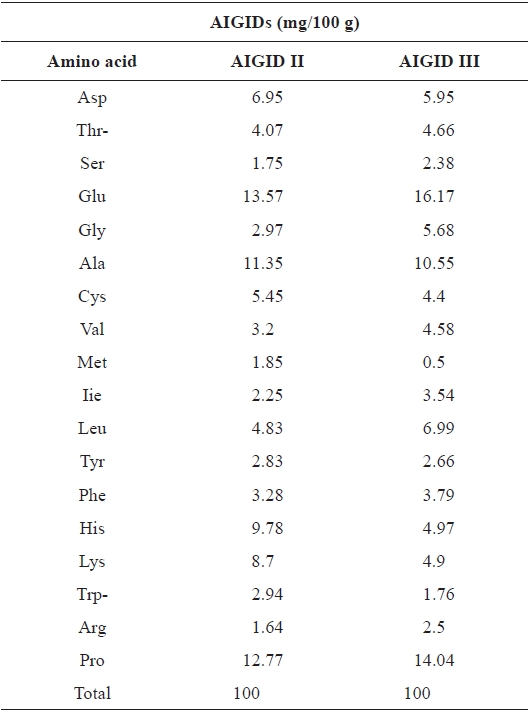
The amino acid compositions of AIGID II and III are shown in Table 1. AIGID II and III were rich in acidic amino acids (glutamic acid, aspartic acid), proline (Pro), alanine (Ala), histidine (His), and lysine (Lys), which constituted 63.12% of the total amono acid residues in AIGID II and 56.58 % of AIGID III. The amino acid composition of a protein is associated with its biological activities; indeed, Glu, Ala, and His have been reported to be important for the inhibition of MMP-2 and -9 (Emara and Cheung, 2006).
In this study, we demonstrated that AIGID II and III markedly decreased MMP expression, cell migration, motility and invasiveness (Fig. 4). Tumor cell invasion of the ECM is an important step in tumor metastasis, and involves the attachment of tumor cells to ECM (Cavallaro and Christofori, 2001; Zhang et al., 2009). Tumor cell migration and invasion are dependent on gelatinase activity. MMP-2 and MMP-9 are important for tumor metastasis. MMPs are highly expressed in human cancers, and a direct relationship between cancer progression and MMP expression and activity has been established by many studies (Rajapakse et al., 2007). The present study confirmed the multi-inhibitory effect of AIGID II and III
on MMP-2 and MMP-9 expression using an artificial substrate and gelatin zymography. In PMA-treated HT1080 cells, AIGID II and III suppressed the expression and secretion of MMP- 2 and MMP-9 by down regulating of MMP-9 transcriptional activation in a dose-dependent manner (Fig. 5). The MMP-2 and MMP-9 promoters both contains NF-κB sites.
[Table 1.] Amino acid composition of abalone intestine gastrointestinal digests (AIGIDs)
Amino acid composition of abalone intestine gastrointestinal digests (AIGIDs)
The human fibrosarcoma HT1080 cell line produces large quantities of MMP-9, whereas normal human fibroblasts have been reported to secrete little or none (Moll et al., 1990). Several studies have shown that PMA can also induce expression of MMP-2 and MMP-9, another important MMP involved in cancer progression. However, the expression level of MMP-2 in HT1080 cells is lower than that of MMP-9 (Kong
Furthermore, the NF-κB family of transcription factors has been shown to be present in most cell types, and speci?c NF- κB binding sites, termed κB sites, have been identi?ed in the promoters of a large number of inducible genes. p50 and p65 are two NF-κB subunits that contain transactivation domains and are necessary for gene induction. NF-κB regulates critical processes in cancers, including invasion, metastasis, apoptosis, angiogenesis, growth, and proliferation (Hayden and Ghosh, 2004; Mendis et al., 2009). NF-κB triggers MMP-2 and MMP-9 gene transcription in response to chemical stimuli, such as PMA, in HT1080 cells (Kim and Kim, 2010). The promoter of the MMP-9 gene contains several putative binding sites for transcription factors that regulate gene expression, an AP-1 binding consensus site at -79 bases upstream from the starter site. Further upstream there is a cluster of regulatory elements, including AP-1, AP-2. and NF-κB binding sites. Additionally, in some inflammatory conditions, MMP-9 transcription can be regulated by NF-κB via different signalling pathways (Hayden and Ghosh, 2004). Thus, we evaluated the ability of AIGID to down-regulate NF-κB pathways. AIGID suppression was clearly dose-dependent. However, it did not suppress PMA-induced translocation of p50, also a component of NF-κB (Fig. 6). MMP-2 and MMP-9 activities were reduced in the presence of some amino acids including cysteine and histidine, cysteine had the strongest inhibitory effect on both MMP- 2 and -9 expression (Emara and Cheung, 2006). Both the differential inhibition of MMP-2 and MMP-9 expression by AIGID we report here and the molecular structure of some amino acids (cysteine and histidine), are consistent with this.
In conclusion, the AIGID II and III fractions of AIGIDs inhibited the PMA-induced invasion and migration of HT1080 cells. Furthermore, AIGID II and III inhibited MMP-2 and MMP-9 expression through down-regulation of the transcription factor NF-κB. Therefore, both AIGID and its active components may be useful as anti-invasive agents in therapeutic strategies against ?brosarcoma metastasis.