An improved strain of the red seaweed Porphyra suborbiculata containing an increased amount of the essential amino acid ?-lysine was obtained through mutation and analog enrichment. Mutagenesis using a 10% lethal dose of ultraviolet irradiation and an enrichment culture with the ?-lysine analog aminoethyl-?-cysteine (AEC) was repeated to select the most productive strain using monospores of P. suborbiculata. The concentrations of AEC required to produce 50 and 100% inhibition of survival were 60 and 115 mM in the parent strain, and 72 and 135 mM in the selected AEC-resistant strain, respectively. The AEC-resistant strain, L130, produced 1.74-fold more lysine compared to its parent strain. Thus, mutagenesis with analog enrichment shows promise for selecting seaweed strains that can overproduce this essential amino acid.
The genus
Bilogy and ecology of
Until now,
As has been demonstrated repeatedly in agricultural crops and other types of cultivation, the genetic improvement of a cultured species is generally crucial to maximize yields and to obtain useful by-products. However, unlike terrestrial plants, improvement techniques for seaweed strains have generally been restricted to classic breeding methods, particularly strain selection. To date, the most successful method for producing new strains of
The amino acid composition of
Juvenile blades of
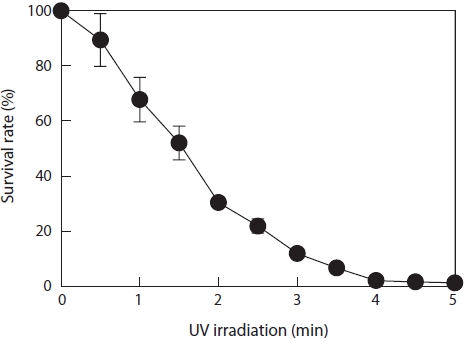
For monospore mutation, ultraviolet (UV) radiation was used as a mutagenic agent. The UV source was a 254-nm wavelength germicidal lamp (30 W) that was placed 20 cm above the monospore-containing plates. To measure the lethal exposure time for the parent monospores, 500 μL of culture media containing approximately 1,000 monospores per well in a 24-well plate was exposed to UV light with continuous agitation; a sample was removed every 30 s. The irradiated monospores were immediately stored in darkness for 1 day to avoid photoreactivation (Carlton and Brown, 1981) and then grown to juvenile blades over a period of 1 week. Survival rate (%) of the irradiated spores compared to nonirradiated spores was calculated as the number of regenerated monospores in PES. The regenerative ability of plants is more severely affected by mutagens more so than growth potential (Moustafa et al., 1989). Thus, we selected a level of mutagenesis that did not significantly inhibit regeneration (30 s of irradiation, which yielded almost 90% survival). A sufficient number of next-generation monospores was viable at this level.
To select lysine overproducers, the UV-treated monospores were grown in PES that contained the lysine analog
Survival rate (%) of the monospores in AEC-containing
PES was calculated as a relative rate: (A/N) × 100, where A = the number of blades that germinated after culture in AEC-containing PES for 1 week and normal PES, and N = the number of blades after culturing in normal PES. Juvenile blades were grown in a temperature-controlled incubator under light at 40 μmol m-2 s-1 and 20℃. The cells of the blades were counted under a microscope with a hemocytometer. The specific growth rate (λ) of the blades was calculated as cell growth against culture time: logN - logN0 = λ(T - T0)/2.303, where N = the number of cells on day T and N0 = the number of cells on day T0.
>
Analysis of gross biochemical composition
Biomass dry weight was measured after washing with 0.5 M ammonium bicarbonate (pH 7.5) and drying at 95℃ for 1 day (Zhu and Lee, 1997). Dried samples were ashed by heating for 5 h in an electric oven at 540℃ (Association of Official Analytical Chemists, 2005). Total carbohydrate content was determined by the phenol-sulfuric acid method (Kochert, 1978) with glucose as the standard. Total lipids were extracted by hexane and isopropanol (3:2) as the solvent (Radin, 1981) and quantified gravimetrically. The amount of soluble protein in the cells was estimated by the method of Lowry et al. (1951) after heating the cell suspension at 100℃ in 1 N NaOH for 2 h to achieve complete protein solubilization. Bovine serum albumin was used as the standard when determining the protein content.
>
Measurement of free amino acids
Dried samples (100 mg) of the blades were suspended in 5 mL of H2O in a hydrolysis tube at 60℃ on a heating block for
1 h. A total of 100 mg of sulfosalicylic acid was added to the homogenates, which were refrigerated (4℃) for 2 h and then centrifuged (15,000 rpm, 4℃, 15 min). The supernatant was collected and evaporated in a rotary vacuum evaporator. The residue was dissolved in 2 mL of Li-citrate buffer (pH 2.2) and filtered prior to measurement through a filter membrane (0.2 μm). Amino acids were analyzed using a Biochrom 20 amino acid analyzer (Cambridge, UK).
Approximately 40 monospores (average size, 15 μm) were produced from an average juvenile blade that was 100 μm in length. Monospores and juvenile blades were separated by filtering through a 20-μm mesh nylon membrane. The conditions for mutagenesis were determined by UV irradiation over various time periods with specimens located 20 cm from a 30-W UV lamp. After irradiation, the monospore survival rate was determined (Fig. 1). Irradiation for 30 s yielded almost 90% survival. Only 10% of the monospores were destroyed by UV irradiation under these conditions; the other 90% were mutated or undamaged. All monospores died after 4 min of irradiation. Thus, we decided to use the conditions that produced a 90% survival rate (30 s of irradiation) for mutagenesis.
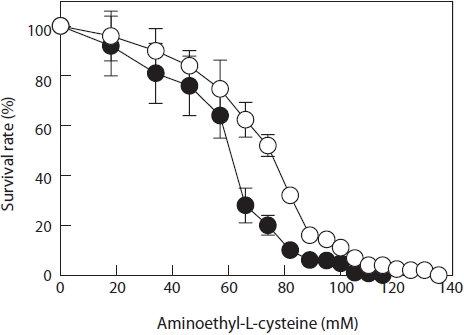
Resistance of the parent monospores to AEC was determined from a dose-response curve (Fig. 2). IC50 was approximately 60 mM and IC100 was 115 mM. Thus, the selection of AEC-resistant mutants after UV treatment commenced with a 115 mM AEC enrichment culture. After culturing the 30-s UV-irradiated monospores in 115 mM AEC for 1 week, the surviving monospores (2nd generation) were placed in fresh
PES without AEC for germination, growth to juvenile blades, and monospore production. These new monospores were mutated again by exposure to UV irradiation for 30 s and then cultured in PES containing 120 mM AEC. After 1 week of treatment, the surviving monospores (3rd generation) were placed in fresh PES to regenerate blades and monospores. These monospores were mutated in the same way and cultured in PES containing 125 mM AEC. After 1 week, the surviving monospores (4th generation) were placed in fresh PES to regenerate blades and monospores. Again, the new monospores were mutated and cultured in PES containing 130 mM AEC for 1 week. The surviving monospores (5th generation) were placed in fresh PES, and one of the rapidly growing blades was selected. The selected juvenile blade was labeled strain L130. Monospores obtained from the 5th generation were mutated and cultured in PES containing 135 mM AEC. No monospores survived at this AEC concentration. The resistance of the L130 monospores to AEC was determined from the doseresponse curve (Fig. 2); IC50 and IC100 had increased to 72 and 135 mM, respectively.
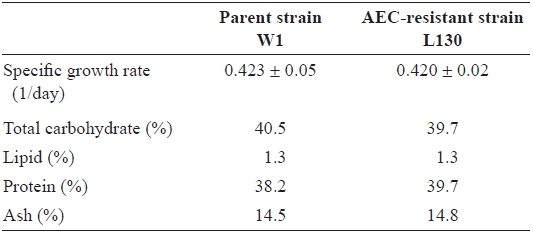
The parent strain W1 and the AEC-resistant strain L130 had nearly the same spore shape (approximately 15 μm in diameter) and blade shape (Fig. 3). The specific growth rates of juvenile blades from strains W1 and L130, measured during
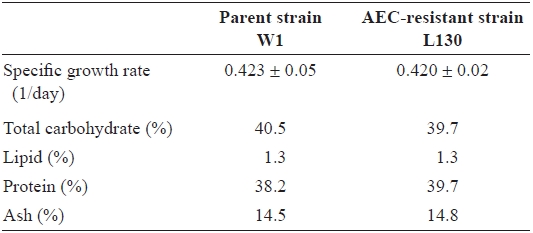
Specific growth rate and gross biochemical composition of juvenile blades of the parent strain (W1) and AEC-resistant strain (L130) of Porphyra suborbiculata*
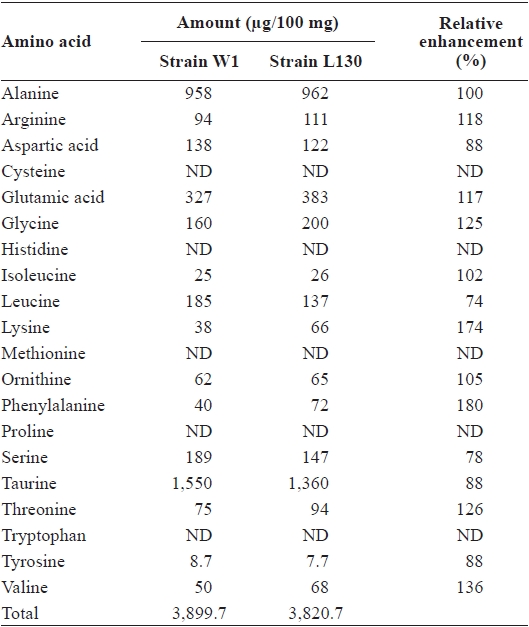
the early growth phase between days 6 and 7, were also approximately the same at 0.42 cm per day (Table 1). In terms of gross biochemical composition, no significant differences were observed between W1 and L130 in total carbohydrate, lipid, protein, or ash content (Table 1). Free amino acid composition was also compared between the parent and AECresistant strains. L130 produced 174% more free lysine than strain W1 (Table 2). Strain L130 also produced 180% more phenylalanine compared to the parent strain, but it produced less leucine and serine. Alanine and taurine were dominant in strain L130 and their levels were comparable to those of the parent strain.
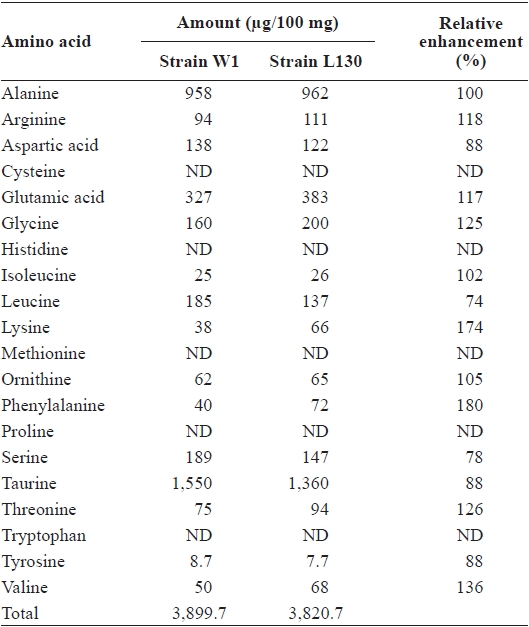
Composition of free amino acids in the parent strain (W1) and AEC-resistant strain (L130) of Porphyra suborbiculata on a dry weight basis
Occurrence of mutations, even when induced by highly efficient mutagens, is relatively infrequent. For example, the mutation frequency in a bacterial population may be as low as 1 per 106 or 107 cells (Carlton and Brown, 1981). Recovering a particular mutant from such a large background of nonmutant cells can be tedious, especially when indirect detection techniques such as amino acid analysis must be employed. In such cases, an enrichment step is often required before mutant screening. The use of amino acid analogs for enrichment does not allow nonmutant cells to grow because of competitive interference with normal amino acids (Queener and Lively, 1986). The production of primary metabolites such as amino acids is regulated by metabolic feedback mechanisms (Kisumi, 1986), including feedback inhibition and repression. The destruction of feedback controls is the most important criterion for the overproduction of a desired amino acid (Kisumi, 1986). Generally, strains in which feedback controls are released can be obtained by isolating amino acid analog-resistant mutants. The lysine analogs AEC and δ-hydroxylysine inhibit the growth of wild-type
Lysine is an indispensable amino acid that is essential in the diet of humans. However,