The world oceans are vast reservoirs of carbon dioxide (CO2) (Feely et al., 2004; Sabine et al., 2004). The atmospheric CO2 concentration has increased rapidly over past decades due to the burning of fossil fuels and other anthropogenic activities (Intergovernmental Panel on Climate Change, 2007). Dissolution of CO2 in seawater shifts the carbonate equilibrium, increasing the H+ ion concentration (i.e., pH) and decreasing the CO3 2- concentration. Increased pCO2 has led to a 0.1 unit decrease in surface ocean water pH over the past 200 years (Caldeira and Wickett, 2003) and it is projected to decrease by 0.3 to 0.4 units by 2100 (Caldeira and Wickett, 2003; Raven et al., 2005). Reductions in seawater pH have detrimental effects on the development and reproductive processes of many marine organisms (Portner et al., 2004, 2005; Raven et al., 2005). Also, increases in CO2 have been suggested to not only affect individuals but also the entire living ecosystem (Widdicombe and Spicer, 2008).
Mimicking ocean acidification (OA) has been difficult to study its effects on ecosystems and animals, although several experimental systems have been designed to estimate the effects of OA. Some groups have designed microcosm systems in which concentrated CO2 was manipulated by air-CO2 gas mixing systems (Findlay et al., 2008; Arnold et al., 2009; Egilsdottir et al., 2009). Other groups have used different molecular acids (H2SO4) to control pH manually, with pH being monitored once a day (Zalizniak et al., 2009). The designs of the pH-controlling systems noted above either require large budgets and space or well-trained workers to obtain reliable data. In the present study, we constructed a pH controller that was a simple, cost-effective, and automatically operated system for maintaining water pH accurately.
Until now, studies on the effects of OA have mainly focused on the growth and development of calcareous marine organisms, including corals (Reynaud et al., 2003; Langdon and Atkinson, 2005), mollusks (Michaelidis et al., 2005; Ellis et al., 2009), echinoderms (Dupont et al., 2008; Havenhand et al., 2008), copepods (Kurihara et al., 2004), and amphipods (Egilsdottir et al., 2009). Since crustaceans are considered one of the most vulnerable groups because of their dependence on the availability of calcium and bicarbonate ions for the mineralization of their exoskeleton. The effects of lower pH (either from direct exposure to HCl or CO2) have been noted on the survival and growth of shrimps
In the present study, we developed a cost-effective pH-control system that can maintain preset seawater pH by automatically regulating CO2 injections. This system was reliable for various animal experiments related to OA. Once the reliability of the system had been confirmed, four different pH treatments (7.0, 7.3, 7.6, and control) combined with three salinity levels (15, 25, and 35 ppt) were maintained and the hatching rate of Artemia was measured.
Commercially available
>
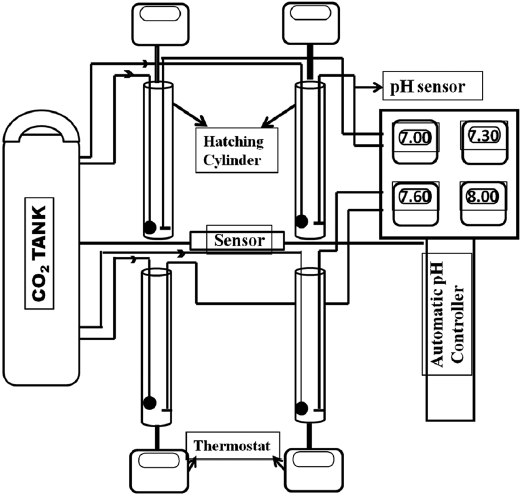
pH-control system using automatic CO2 injection
A CO2 control system was constructed by assembling a CO2 gas tank, pH sensors, a pH monitor, and an automatic pH controller. Fig. 1 depicts how different pH levels were maintained over the experimental period by the automatic pH controller. The pH level was set at the onset of an experiment and a pH sensor was placed in each hatching cylinder (1 L). Another sensor from the pH controller was linked to the CO2 container and regulated the amount of CO2 injected into the water. A solenoid valve connected to the pH controller allowed the CO2 input to be switched on and off automatically to achieve a constant pH, which could be checked with the pH monitor.
>
Measurement of the hatching rate
An experiment was conducted to investigate the effects of pH changes that were induced by CO2 and salinity on
7.0, 7.3, and 7.6. Untreated seawater was used as a control (pH 8.0 ± 0.1). Three replicates were conducted for each experimental treatment. One gram of
To confirm the reliability of the pH-control system as described above, blanks without
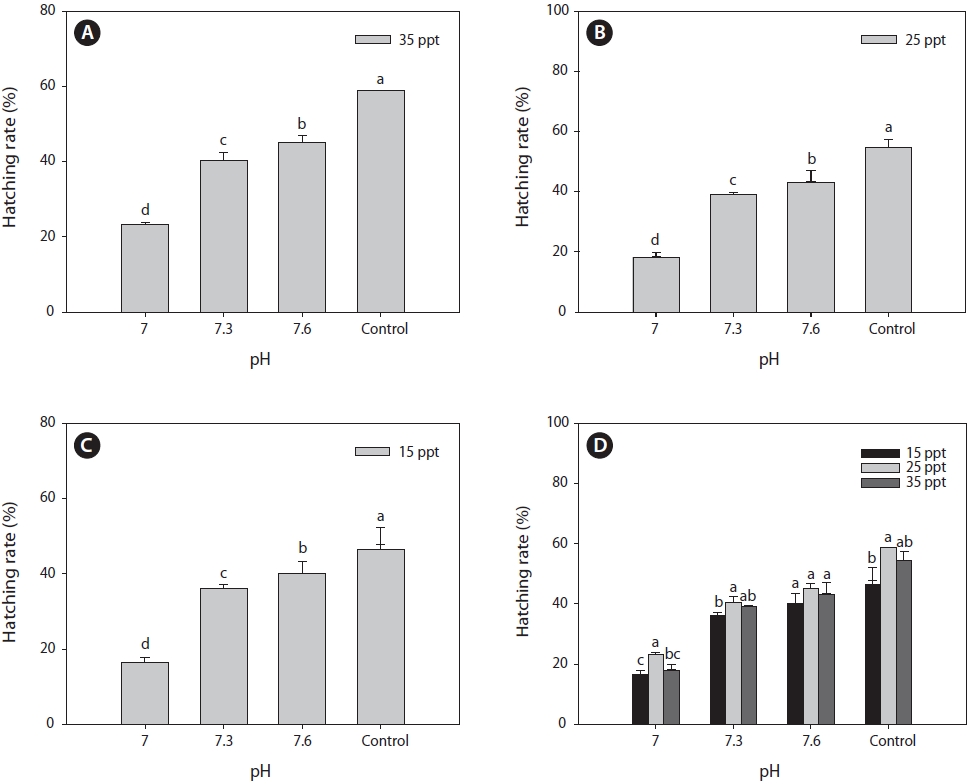
After 24 h of cyst incubation, CO2-mediated pH changes had significant effects on the hatching rate of
maximum hatching rate was observed with the control pH (~8.0) and decreased significantly to the lower pH (7.0), irrespective of salinity (Fig. 2A-2C). At a salinity of 15 ppt, the mean hatching rate decreased to 17% with pH 7.0, whereas it was 46% with the control pH (~8.0). Both the 25 and 35 ppt salinity treatments exhibited similar patterns of decreased hatching rates, dropping to 22% and 18%, respectively, in the lowest pH (7.00) treatment. Hatching rate decreased by 2.7, 2.5, and 2.9 times compared to the control pH with salinity levels of 15, 25, and 35 ppt, respectively (Fig. 2D). A study on the effects of H2SO4 acidified seawater on the hatching and survival of
In this study, the hatching rate of
In conclusion, we constructed a cost-effective pH-control system involving automatic CO2 injection that could be used to study the effects of CO2-induced reductions in pH on marine animal physiology. We also demonstrated that pH and salinity changes had independent effects on the hatching rate of