Recent advances in mass spectrometry (MS) are promoting use of the technology in applications outside traditional laboratory settings. The two major areas of research that are driving these non-traditional MS applications are; (i) the miniaturisation of MS instruments and (ii) the development of ambient ionisation techniques. The deveopment of both portable1 and handheld2 systems as well as a commercialised field-ready mass spectrometer3 represent the progress made in MS miniaturisation over the past 5 years. These advancements have finally enabled mass spectrometric analysis for some highly desirable applications,
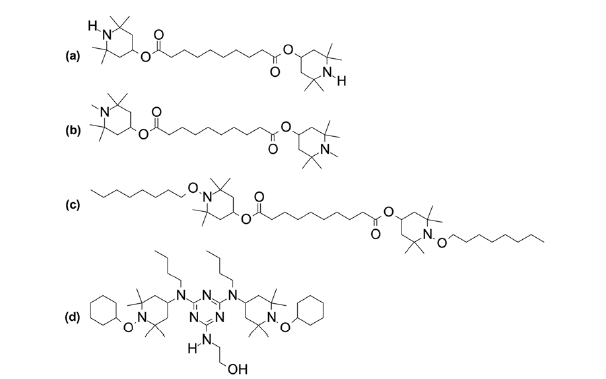
Ambient ionisation mass spectrometry has been shown to be effective for the direct analysis of a wide range of compounds within chemical,9,10 biological11-13 and forensic applications.14,15 Recently, the ambient MS methods of desorption electrospray ionisation (DESI) and liquid extraction surface analysis (LESA) have been employed for the analysis of polymers, including the detection and characterisation of hindered amine light stabilisers (HALS) in thermoset polyester-based coil coatings.16,17 HALS represent a class of polymer additive that act as free radical scavenging antioxidants present in the formulation of many plastics and surface coatings.18 Initial oxidation of the hindered amine yields a stable nitroxyl radical that is proposed to react with macro alkyl radicals generated
While DESI and LESA have both demonstrated their use as potent analytical tools they both require specialised ion source configurations. In collaboration with our industry partners our aim is to develop a simpler, more robust method suitable for process monitoring that does not require such specialised equipment. Ideally, this method should be able to be carried out on any mass spectrometer with an atmospheric pressure ion source interface. The present study reports a method that builds on the paper spray ionisation method developed by the research groups of Cooks, Ouyang and co-workers at Purdue University (West Lafayette, Indiana, USA).19,20 The direct sampling method, reported herein as ‘Paint Spray’, shares characteristics of both ESI and the ambient ionisation methods and is useful for fast, qualitative analysis of polymer-based surface coatings on conductive surfaces. Analyte desorption is achieved by liquid extraction of analytes at or near the surface, and a high electric field is used to facilitate ionisation. Pneumatic assistance is not required to transfer the analyte: a voltage is simply applied to the wetted metal substrate, which is positioned in front of a mass spectrometer.
Methanol and formic acid were high performance liquid chromatography grade (Crown Scientific, Minto NSW, Australia). The hindered amine light stabilisers TINUVIN® 770 (bis (2,2,6,6-tetramethyl-4-piperidinyl) sebacate), TINUVIN® 292 (bis (1,2,2,6,6-pentamethyl-4-piperidinyl) sebacate), TINUVIN® 123 (bis (1-octyloxy-2,2,6,6-tetramethyl-4-piperidinyl) sebacate) and TINUVIN® 152 (2,4-bis(
>
Preparation of coated steel panels
The topcoat paint system employed in these studies was a solvent-borne, polyester topcoat paint incorporating a melamine-formaldehyde cross-linker, acid catalysed and
formulated for coil paint-line application. As a wet paint, this sample was found to be 40% w/w resin solids by thermogravimetry (Perkin-Elmer TGA 7, Perkin-Elmer Co. Ltd., Norwalk CT, USA). A bulk sample of this paint was sub-sampled and weighed into small containers with HALS added at
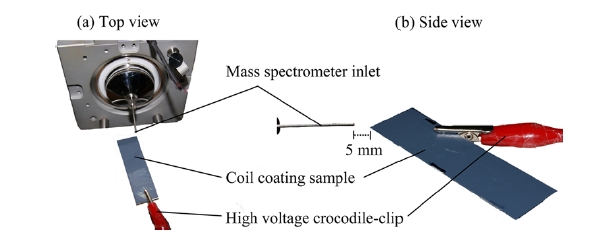
All experiments were carried out with a ThermoFinnigan LTQ 2-dimensional (2-D) linear ion trap mass spectrometer (ThermoFisher, San Jose CA, USA). Instrumental conditions used for positive-ion mode mass spectrometry: Paint Spray voltage: 5 kV; capillary temperature: 200℃; heated-capillary voltage: 30 V; tube-lens voltage: 100 V. Paint Spray-MS/MS spectra were acquired under the same experimental conditions with a precursor ion isolation width of 1.5 Da and normalised collision-induced dissociation energy of 30 (arb. units). Metal panels with a thermosetting polyester-based coating were fixed in position in front of the mass spectrometer inlet with a corner directed toward the extraction orifice. Small volumes of methanol (10 μL) acidified with formic acid (0.1% v/v) were deposited on the coating at the point closest to the mass spectrometer and a high voltage (5 kV) was applied to the coating using a crocodile-clip connection (Figure 2).
Figure 2 shows the experimental set-up for Paint Spray-MS with samples connected to a high voltage using a crocodile-clip and mounted in front of the MS inlet. Preliminary Paint Spray-MS analysis of coatings before wetting the surface with acidified methanol yielded no detectable signal for HALS from the sample. Following addition of solvent,
however, Paint Spray-MS provided rapid detection of HALS from the sample with high signal intensities. Samples were positioned on-axis with the MS inlet at a distance of 5 mm with no signal detected by the mass spectrometer at distances > 10 mm. The corner of the sample was directed at the MS inlet as the high electric potential applied between the sample (5 kV) and the MS inlet (30 V) generates an electric field that induces a charge that accumulates at the apex of the sample. An insulating substrate (
The addition of solvent to the samples surface coincided with the production of a visible droplet stream similar to that produced by the Taylor cone observed under electrospray ionisation conditions. The potential difference and pressure gradient serve to draw the charged droplets into the mass spectrometer with the transition from atmospheric pressure to high vacuum inside the mass spectrometer enough to facilitate analyte desolvation. A constant droplet stream was generated for up to 30 seconds with 10 μL of solvent deposited with droplet generation and analyte detection reproducible on the addition of subsequent 10 μL aliquots of solvent.
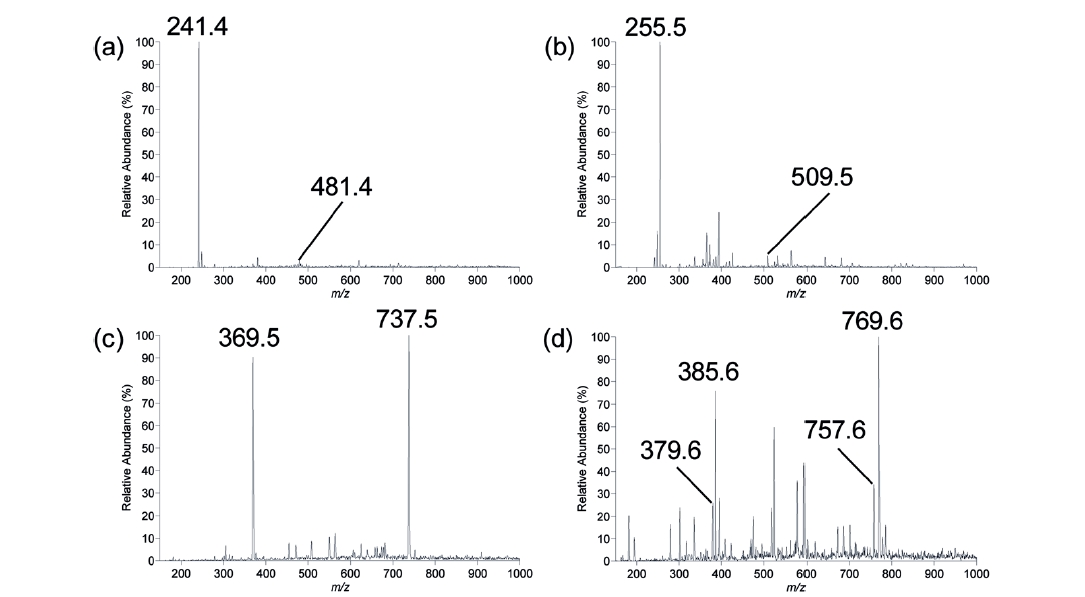
Positive ion Paint Spray-MS spectra of four polyester-based coil coatings each containing a different commercially available hindered amine light stabilisers are shown in Figure 3. The spectra yield intense signals for singly and/or doubly charged ions arising from protonation of the heterocyclic nitrogen(s) within the HALS compounds. In comparison with other ambient mass spectrometric methods employing solvent based desorption, Paint Spray-MS spectra closely resemble those acquired using LESA and differ to that of DESI in terms singly/doubly charged ion abundance ratios. This can be rationalised by the relatively large amount
of solvent involved with LESA and Paint-Spray-MS (2-10 μL) compared with DESI (microdroplets). Figure 3(a) shows the positive ion Paint Spray-MS spectrum for a thermoset coil coating formulated with TINUVIN® 770. The spectrum exhibits a base peak at
Figure 3(c) shows the positive ion Paint Spray-MS spectrum for a thermoset coil coating formulated with TINUVIN® 123. The spectrum exhibits two major peaks at
The three stabilisers reported thus far can all be considered as ‘migratory’ HALS, able to move freely through the polymer matrix when the polymer is above its glass transition temperature. Above this temperature, the polymers physical properties become altered, changing from a hard and relatively brittle state into a molten or rubber-like state. Figure 3(d) is a positive ion Paint Spray-MS spectrum of the ‘non-migratory’ HALS TINUVIN® 152. This compound is designed to co-condense with the polymer backbone through condensation of the primary alcohol substituent to melamine and isocyanate cross-linkers and therefore should not be able to be detected under normal ambient ionisation conditions. However, Figure 3(d) exhibits ions at
Paint Spray ionisation has been developed for direct MS analysis of polymer-based surface coatings requiring no sample pre-treatment. Its capabilities have been demonstrated with the analysis of four commercially available HALS (three migratory and one non-migratory) present in polyester-based thermoset coil coatings. For the three migratory HALS, the singly or doubly charged ion of the parent HALS compound were detected as the base peak, being clearly distinguishable within the spectrum. For the non-migratory HALS (TINUVIN® 152), the [M+H]+ ion of a synthetic by-product unable to co-condense with the polymer was detected as the base peak. Unreacted TINUVIN® 152 present in the coating was also detected as both a singly and doubly charged ion. The technique has proven to be rapid and simple to employ making it ideal for use by those outside the research laboratory setting. The potential of Paint Spray ionisation when combined with a portable or handheld mass spectrometer for mass analysis expands the possibilities for MS, such as on-line quality control within the surface coating industry. For instance, the degree of cross-linking of non-migratory HALS could be monitored by the detection of the unbound material present in the coating.